1. Background
Depression will account for the second largest cause of disease burden by 2020 (1). In addition, two-thirds of all deaths, disability-adjusted life years, and the burden on the national health care system are mostly linked to chronic diseases worldwide (2). Obviously, chronic diseases and their treatment affect the patient's physical and mental health so that the evaluated risk of depression is reported between 8.3% among diabetic and 44% in kidney disease patients (3, 4). In some studies, symptoms of depression were reported twice more than the adults in the general population (5). These patients have a lower health status and more complications, mortality, and medical costs, too (6).
Since the chronic disease has a long period and incomplete cure, patients should be able to manage their disease to have a good quality of life. The patients with chronic illness should be considered on self-management. In fact, the outcome of chronic illness depends on effective self-management (7).
Self-management means “the tasks of having confidence in patients to deal with medical management, role management, and emotional management of their conditions” (8).
On the other hand, psychological factors can affect self-management behaviors such as medication adherence (9), physical activity (10), medical appointments (11), and help-seeking (12). In addition, clinician-patient communication is restricted in the presence of depression in these patients (13).
To the best of our knowledge, in spite of increasing chronic diseases in our country, the prevalence of depression and its association with chronic diseases have been discussed a little. In addition, available studies have focused on depression only among diabetic (14), renal (15, 16), or heart disease (17) patients separately. However, so far, very little attention has been paid to patients with various types of chronic diseases simultaneously.
This paper seeks to address the following questions: (1) the effect of the severity of depression on self-care behaviors (physical activity, medication adherence) and self-management (communication with the physician or other health care professionals, and daily activities among the outpatients), (2) the association between demographic and contextual variables (age, sex, duration of illness, type of disease, marital, and economic status) and depression, and (3) the differences among the severity of physical symptoms such as pain in the presence of depression in the government healthcare centers in the urban areas of Isfahan, Iran.
2. Objective
The aim of the present study is the Explain the relationship between severity of depression and self-care behaviors in Iranian patients with chronic diseases.
3. Materials and Methods
3.1. Subjects
The present study was a cross-sectional study. The statistical population of this study included patients with any kind of chronic diseases admitted to the government healthcare centers of urban areas of Isfahan, Iran. These patients included middle-aged individuals or elders that registered as chronic disease patients in these centers. Based on the national care protocols, their health history was recorded in the health records and the presence of the disease was approved by the physicians of the health care centers to receive care and treatment. According to the Cochrane formula by considering the respective levels of confidence interval and probability (0.95 and 0.5), the error level of 0.04, and the power statistical test of 80%, the sample size was calculated as 496. The sample was selected based on a two-step method. First, six healthcare centers were selected randomly. Since the number of registered patients in each healthcare center was different, 25 - 50 patients having the inclusion criteria were selected randomly to participate in the study from every healthcare center. The inclusion criteria were having a chronic illness at least for six months ago, having the prescription of medication, willingness to take part in the survey, and having the ability to walk. The patients with contradictory answers due to geriatric problems and distorted questionnaires were excluded so that 13 patients were omitted from the study.
After obtaining an ethical approval from the ethics committee of Isfahan University of Medical Sciences (reference: 393790.12/1/2014), coordinating with the related units, and obtaining necessary permissions, informed consent forms were filled out by the patients. They were assured that the questionnaires were anonymous so that the number and the type of disease would be held confidential. Questionnaires were completed during interviews in a quiet room (the majority of patients had not adequate ability to fill out the questionnaires because of illiteracy or vision problems). To avoid bias, one trained questioner conducted all of the interviews.
3.2. Study Tool
We measured social and demographic data with a set of questions related to age, marital status, sex, education level, weight, height, type of disease (diabetes, hypertension, heart disease, osteoporosis, arthritis, cancer, hypothyroidism, renal disease, and so on), and duration of illness. Their weight was recorded in kilograms using a calibrated digital scale. Participants were weighed without their shoes. Their heights were obtained by taking the average of two readings of height in meters using a portable stadiometer. Body mass index was determined by dividing weight (kg) by height squared (m2).
The translated version of “Stanford Self-Management questionnaire in chronic disease patients” was used. This instrument was developed and validated by the Stanford Patient Education Research Center. The questionnaire evaluates concepts such as self-efficacy, communication with health care workers, social/daily role activity limitation, physical symptoms, and other self-care activities related to chronic illness (18). We followed the translation-back-translation process. Then, nine experts were invited to assess the face and content validity indices. The Persian version of the questionnaire was revised according to their comments. After the pilot test, the reliability coefficient of the total scale was α = 0.79 with the internal reliability of 0.76. The questionnaires were filled out by 30 patients with at least one chronic disease. The reliability coefficient of the total scale was α = 0.79; then, these patients were omitted from the main study (19).
Depression was evaluated by the nine-item Patient Health Questionnaire (PHQ-9) as an easy-to-use and acceptable instrument for depression measurement. The questionnaire scores each of the nine DSM-IV criteria for depression as “0” (not at all) to “3” (nearly every day). PHQ-9 scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively (20). Internal reliability has been reported with Cronbach’s alpha of 0.89 (18).
The Visual Analogue Scale for Pain is a numeric scale to evaluate physical symptoms (pain) intensity and its internal consistency was 0.86 among patients with a kind of chronic illness (21).
3.3. Social/Daily Role Activity Limitation
Four questions measured the effects of illness on daily activities such as social relationships with family, neighbors, friends, and other impacts of the illness on hobbies, the indoors duties and outside house tasks during the two past weeks. The obtained internal correlation for the scales varied from 0.70 - 0.75 (18).
The respondents were asked to answer several questions about patient self-care behaviors, including:
1. Physical activity: Did you participate in walking or other aerobic exercises as an exercise during the two past weeks (not as leisure time activity). The choices included never, < 30, 30 - 60, 60 - 180, and more than 180 minutes with the respective scores of 0 - 4 (19).
2. Healthy eating was assessed by the question regarding the consumption of 5 fruits and vegetable servings and eating fat or fried meals daily that scored as “0” (not at all) to “5” (always) (19).
3. Medication Adherence was measured by a 4-item questionnaire. These questions were related to forgiveness and suspicion of drug use discontinuation due to healing or worsening of general health during the two past weeks. The items scoring was based on yes (1) or no (0) (21).
Data were analyzed using Statistical Package for the Social Sciences (SPSS; version 17, SPSS Inc., Chicago, IL). Descriptive statistics were used to establish the frequency, range, mean, and standard deviation (SD) of demographics and physical symptoms (pain, stress) in depression and non-depression groups. The Chi-square test was used to evaluate differences in qualitative variables (gender, economic status, marital status, age, and education level) and self-management behaviors (physical activity, medication adherence, healthy nutrition, and communication with physicians (or health care professionals) in terms of depression. Spearman correlation was undertaken to examine relationships between depression, demographics, self-care duties, and social/daily role activity.
4. Results
From among eligible patients approached, 483 subjects were successfully recruited with a response rate of 97%. Three percent were excluded from the study due to the questionnaire’s distortion. The age range of participants was 30 - 76 years, with a mean of 55.15 (7.21) years and more than half of them were older than 50 years. Nearly 90% of the respondents were overweight or obese. The duration of disease was between 6 months and 45 years with a mean of 8 years for 1 - 5 chronic illnesses. Two-thirds of the participants had approximately more than one chronic disease and their economic status was moderate. Other demographic characteristics are presented in Table 1.
| Factors | Total (N = 483)a | Non-Depression (N = 404)a | Depression (N = 79)a | P Valueb |
|---|---|---|---|---|
| Sex | 0.003b | |||
| Female | 412 (85.3) | 336 (83.2) | 76 (96.2) | |
| Male | 71 (14.7) | 68 (16.8) | 3 (3.8) | |
| Age, y (mean ± SD) | 55.15 ± 7.21 | 55.53 ± 7.02 | 55.28 ± 7.92 | 0.5 |
| BMI (mean ± SD) | 29.71 ± 4.37 | 29.59 ± 4.28 | 30.39 ± 4.78 | 0.136 |
| BMI ranges | 0.553 | |||
| 18.5 - 24.99 | 51 (10.6) | 43 (10.6) | 8 (10.1) | |
| 25 - 29.99 | 226 (46.8) | 193 (47.8) | 33 (41.8) | |
| < 30 | 206 (42.7) | 168 (41.6) | 38 (48.1) | |
| Self-evaluation of economic status | 0.005b | |||
| Bad | 95 (19.7) | 69 (17.1) | 26 (32.9) | |
| Moderate | 350 (72.5) | 301 (74.5) | 49 (62.0) | |
| Good | 38 (7.9) | 34 (8.4) | 4 (5.1) | |
| Education status | 0.219 | |||
| Illiterate | 176 (36.4) | 144 (35.6) | 32 (40.5) | |
| Under 12 years of education | 233 (48.2) | 192 (47.5) | 41 (51.9) | |
| 12 years of education | 58 (12) | 53 (13.1) | 5 (6.3) | |
| Academic education | 16 (3.3) | 15 (3.7) | 1 (1.3) | |
| Marital status | 0.7 | |||
| Married | 432 (89.4) | 362 (89.6) | 70 (88.6) | |
| Divorced or widow | 79 (16.4 ) | 70 (17.6) | 9 (16.2) |
aValues are expressed as No. (%) unless otherwise indicated.
bThe significance level of Comparison between the two groups of depression and non-depression.
Based on the self-report questionnaire, the majority of participants (85.2%) were not depressed and 14.7% were depressed. Twenty six cases (5.4%) had moderately severe or severe depression. Majority of the depressed participants were female and only 8.3% of them were male (P < 0.05). The mean age of the patients with depression was significantly lower than that of the non-depressed patients (P < 0.05).
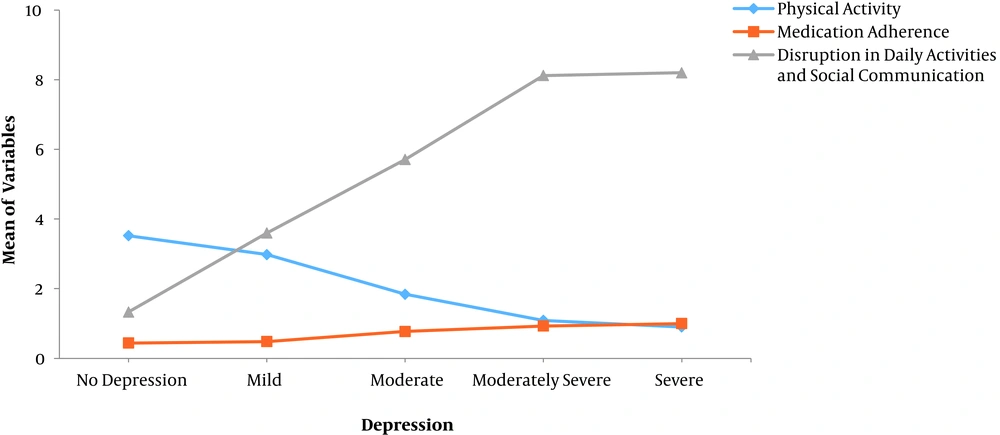
The Chi-square test showed that there was a significant relationship between self-care behaviors such as physical activity (P < 0.05) and medication adherence (P = 0.001), and depression. In addition, the relationship between depression and social role activity was significant (P < 0.001). There was a relationship between depression and self-care activities so that the more severity of depression, the less physical activity and medication adherence. There was a significant relationship between other behaviors such as social/daily role activities and depression, too. Nearly 20% of the patients had a weak medication adherence because of forgiveness or doubt to take medication. In addition, disruption in social role activity increased with the severity of depression (Figure 1). There was no significant relationship between healthy eating and depression (Table 2). In our study, one-fifth of these patients had problems in communication with family, relatives, and doing their household chores.
There was a significant relationship between the presence of diseases such as rheumatoid arthritis or other skeletal diseases (osteoporosis (and depression. Rheumatoid arthritis patients were more likely to report depression (OR = 4.41, 95% CI 0.47 - 1.38, P < 0.05) that was the case of skeletal disease patients (OR = 1.70, 95%CI 1.03 - 2.77, (P < 0.05). In other words, the risk of depression in rheumatoid arthritis and skeletal disease patients was 4.41 and 1.70, respectively, which was greater than the risk of depression in other chronic disease patients. Overall, the level of pain was greater among depressive patients than among non-depressive patients (Table 3).
| Self-Care Activities (Past Two Weeks) | Non-Depression (N = 404)a | Depression (N = 79)a | OR (95% CI) | P Value |
|---|---|---|---|---|
| Five servings of fruits and vegetables daily | 395 (97.8) | 78 (98.7) | 1.77 (0.22 - 14.23) | 0.583 |
| Eating fat or fried meals | 177 (43.8) | 27 (34.2) | 0.67 (0.40 - 1.10) | 0.113 |
| Physical activity (30 - 60 min/day) | 75 (18.6) | 6 (7.6) | 0.36 (0.15 - 0.86) | 0.017 |
| Medication adherence | 275 (68.1) | 39 (49.4) | 0.46 (0.28 - 0.74) | 0.001 |
| Disruption in daily activities and social communication | 33 (8.2) | 31 (39.2) | 7.26 (4.08 - 12.90) | < 0.001 |
Abbreviation: CI, confidence interval.
aValues are expressed as No. (%).
| Factors | Total (N = 483)a | Non-Depression (N = 404)a | Depression (N = 79)a | OR (95% CI) | P Valueb |
|---|---|---|---|---|---|
| Name of diseasec | |||||
| Hypertension | 359 (74.3) | 303 (75.0) | 56 (70.9) | 0.81 (0.47 - 1.38) | 0.444 |
| Diabetes | 283 (56.6) | 239 (59.2) | 44 (55.7) | 0.87 (0.53 - 1.41) | 0.568 |
| Osteoporosis | 144 (29.8) | 115 (28.5) | 29 (36.7) | 1.46 (0.88 - 2.42) | 0.143 |
| Heart disease | 124 (25.7) | 98 (24.3) | 26 (32.9) | 1.53 (0.91 - 2.58) | 0.107 |
| Renal disease | 73 (15.1) | 58 (14.4) | 15 (19.0) | 1.39 (0.75 - 1.62) | 0.293 |
| Arthritis rheumatoid | 36 (7.9) | 22 (5.4) | 16 (20.3) | 4.41 (2.19 - 8.85) | < 0.001 |
| Skeletal disease | 164 (34.0) | 129 (31.9) | 35 (44.3) | 1.70 (1.03 - 2.77) | 0.034 |
| Duration disease, y | 8.06 ± 6.36 | 8.01 ± 6.23 | 8.32 ± 7.05 | 1.01 (0.97 - 1.05) | 0.697 |
| Pain (0 - 10) | 4.93 ± 3.69 | 4.46 ± 3.64 | 7.34 ± 3.01 | 1.27 (1.17 - 1.38) | < 0.001 |
Abbreviation: CI, confidence interval.
aValues are expressed as No. (%) or mean ± SD.
bThe significance level of comparison between the two groups of depression and non-depression.
cSome of patients had two or more diseases.
There was a significant relationship between economic status and depression (P < 0.05), so that greater percentage of patients with an unfavorable economic level reported a higher level of depression (P < 0.05) (Table 1).
5. Discussion
This study was conducted among 489 outpatients in government health care centers of urban areas of Isfahan, Iran. In our study, there was a significant relationship between depression and self-management behaviors (medication adherence, physical activity, social/daily role activity) among chronic disease patients. The remarkable point is that increased depression severity is followed by weak self-care behaviors such as physical activity. Similar results have been reported by other researchers (22-24). Depression is also known as a mediator for medication adherence in diabetic and hypertensive patients (25, 26). It seems the nature of prolonged diseases, higher perceived barriers, the outlook of hopelessness, and lack of energy and concentration among chronic disease patients can affect medication adherence. Therefore, medication adherence is low in these patients.
There was no association between healthy nutrition and depression in the current study. However, the relationship between depression and a dietary pattern containing vegetables, fruits, and the low-fat food was established in non-patients (25, 26) so that some studies pointed to the protective role of vegetables against depressive symptoms (27). Nevertheless, Rivera-Hernandez’s study was in line with our study (28). This difference may be related to the type of questionnaire used while Jacka et al. (27) investigated the dietary pattern with an accurate instrument of more than 100 food items (28). On the other hand, careful consideration of nutritional patterns among patients is required to respond to this difference.
In our study, there was a relationship between pain and other painful diseases such as rheumatoid arthritis and other skeletal diseases. Studies have found that patients with more frequent pain episodes or longer pain duration are several times more likely to be depressed compared to other patients (29). Major depressive patients had a higher level of pain than non-depressive patients (29). In studies, depression was known as a strong predictor of the onset of an episode of intense low back pain (30). As the history of depression was not discussed in our study, we cannot exactly determine whether pain leads to depression or vice versa.
Severe depression was seen in 5.4% of the participants and major depression in 8.3% by Li (3). Kaur et al. pointed to a similar prevalence among diabetic patients (31). This is while the higher rates of depression have been reported by Sharif et al. (16) and Abbas Tavallaii et al. (15). This difference may be attributed to different instruments and sample sizes that included hospitalized patients with heart or renal diseases. In the current study, the majority of participants were diabetic or hypertensive outpatients without serious complications. In the present study, one-fifth of the participants complained especially of the limitations that prevented from the achievement of household tasks and restrictions in social relationships. Other studies are in line with our study so that the presence of depression in hypertensive patients even without depression symptoms was followed by a higher level of disability (32). In fact, the restriction of social role-related activities is attributed to negative feelings induced by the nature of the long-term disease. The women and patients with low economic status were more depressed than the others in this study. Other studies revealed that sex, low socio-economic class, and marital status were the predictors of depression, too (3, 32, 33).
Definitely, adherence to treatment advice in chronic diseases can decrease the mortality and morbidity of the disease. On the other hand, the influence of psychological factors is emphasized in a better self-care.
5.1. Restriction of Study
Sampling from healthcare centers may be thought to limit the generality of the findings. We had no prior history of depression or antidepressant consumption in patients, too.
5.2. Conclusions
We found a positive association between depression and self-care activities in Iranian patients with chronic diseases. Psychological treatment for depression should be considered to improve the prognosis of chronic diseases.