1. Background
Major depression is a severe chronic mental illness that affects a substantial proportion of the world’s population (1). It is the leading cause of disability and imposes a significant financial burden on society (2, 3). Although several treatment options either pharmacological or psychological are effective for major depression, antidepressant medications remain the mainstay of management in the treatment of patients with moderate-to-severe major depression (4, 5). However, because antidepressant agents achieve clinical improvement in only 65 - 70% of patients, and may cause various adverse effects, the therapeutic approach to depression is still a challenge for clinicians (6, 7). For this reason, newer antidepressants have been developed to improve efficacy and tolerability (8). Citalopram and venlafaxine are among these agents that are generally considered more targeted agents. Citalopram is the most selective and potent Selective Serotonin Reuptake Inhibitor (SSRI) with well-documented antidepressive effects (9). Selective serotonin reuptake inhibitors are effective, well-tolerated and safe antidepressant agents that have been widely prescribed as a first-line medication for major depression (10, 11). Venlafaxine is the first serotonin-norepinephrine reuptake inhibitor antidepressant that has well-documented efficacy against its low tolerability in the acute phase treatment of major depressive disorder (12, 13). Although several investigations have evaluated the efficacy of venlafaxine compared with SSRIs, they have reported controversial results (14). In the light of the above, this study was conducted to compare the efficacy and tolerability of venlafaxine Extended Release (ER) with those of citalopram in patients with major depression.
2. Objectives
There are many antidepressant medications with different side-effects and efficacy profiles. In this study, we compared the efficacy of citalopram and venlafaxine in major depression, which has not yet been studied in Iran.
3. Materials and Methods
3.1. Study Population and Design
This study with the IRCT (Iranian Registry of Clinical Trials) registration code of “201203048513N1” was approved by the Ethic Committee of Shahid Sadoughi University of Medical Sciences, Yazd, IR Iran. After obtaining an informed consent, this double-blind, randomized clinical trial was performed on adults referred to the psychiatry outpatient clinic in Yazd City, the central part of Iran, between March 2011 and December 2012.
3.2. Participants
Among all patients referred to psychiatrists in outpatient services, patients who were diagnosed with moderate or mild major depression were considered as the participants after obtaining an informed consent.
3.3. Intervention
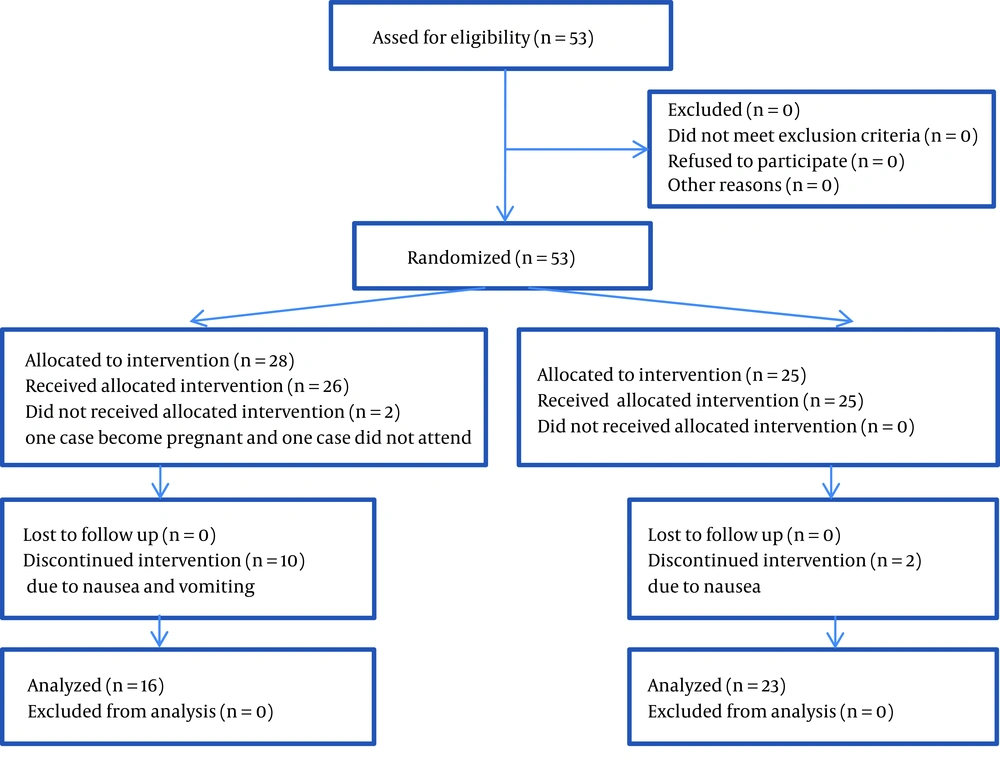
Due to the different shapes of citalopram and venlafaxine ER tablets/capsules, a pharmacologist prepared capsules containing the specific doses of citalopram or venlafaxine ER. All capsules had a similar shape. According to the study group and medication dosage, patients of both groups received capsules in similar packages every 2 weeks. Citalopram was commenced with the initial dose of 10 mg daily, and gradually increased to 40 mg daily. Venlafaxine ER starting dose was 37.5 mg daily; then, gradually increased to 150 mg a day. During the study, one patient stopped taking venlafaxine ER due to an unplanned pregnancy, and another patient left the study due to personal issues and 12 patients stopped using medication due to severe nausea and vomiting.
3.4. Sample Size
Considering the degree of freedom of 80, confidence interval of 95 and standard deviation of 6.5 in Hamilton Depression Rating Scale (HDRS) questionnaire, a sample size of 53 patients was appropriate to be enrolled in this study.
3.5. Randomization
Out of all the patients referred to outpatient clinic, everyone who was diagnosed as a case of moderate to mild major depression by a psychiatrist was included as a participant and allocated into one of the two groups of citalopram or venlafaxine based on the order of their even or odd waiting list number. Both patients and psychiatrists were blind to the type of treatment. Altogether 12 patients stopped participation due to nausea and vomiting and 2 patients decided to not continue participation because of their personal reasons. Finally, 39 patients completed the study, 16 patients in the venlafaxine and 23 patients in the citalopram group. The researcher who was responsible for the evaluation and follow-up of the change in signs and symptoms of depression was blind to the kind of medication and patients were also blind to their drug type (Figure 1).
3.6. Measures
1. Patients were included as study participants if they fulfilled the criteria of major depressive disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). 2. The 17- item HDRS was used for evaluation of depression severity during the course of treatment. Specificity of HDRS was 90 - 94% and the reliability was 84 - 90% (15). 3. A side-effect checklist was prepared according to all side-effects of both citalopram and venlafaxine drugs.
3.7. Exclusive Criteria
Any patient who has been treated with antidepressant medication during the past 4 weeks or having suicidal ideation or attempt, any individual with any other mental disorder or medical illness and pregnant patients were excluded from the study.
3.8. Inclusive Criteria
Any patient aged between 18 and 54 years old, diagnosed as a case of moderate to severe major depression by the staff psychiatrist according to the DSM-IV-TR criteria for major depression was included as a participant.
3.9. Data Collection and Follow-Up
Patients’ information was collected using three questionnaires including a demographic data questionnaire, HDRS and antidepressant side-effect check list. A pretreatment HDRS score was determined for all the participants during the first visit. Then, follow-up visits were arranged on biweekly basis for 8 weeks. On each follow-up session, patients were asked about any possible adverse effects according to the checklist, and an HDRS total score was calculated. All questionnaires were completed by a psychiatry resident who was blind to the type of treatment.
3.10. Statistical Method
Statistical analysis was performed using the SPSS for Windows (version 20.0, SPSS Inc., Chicago, IL, USA). Independent Mann-Whitney U-test and Fisher's exact test were used to analyze the data. P values < 0.05 were considered statistically significant.
4. Results
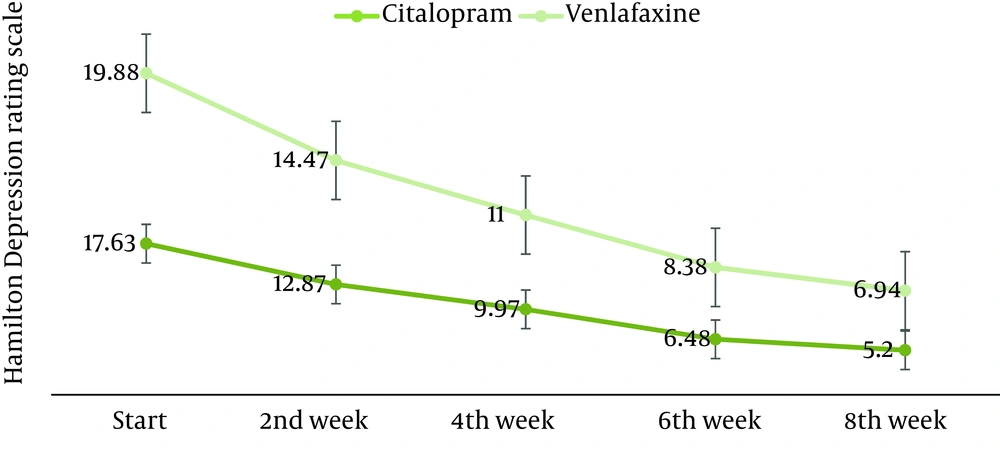
Mean of the age, distribution of sex, and the mean of the duration of depression have been shown in Table 1 based on the treatment group type. Post treatment HDRS score was significantly lower than the pretreatment score in both citalopram and venlafaxine ER-treated group (Figure 2). However, there was no significant difference between two groups regarding the pretreatment and post treatment HDRS scores (Table 2). Furthermore, HDRS scores were analyzed biweekly in each treatment group using an independent sample Mann-Whitney U-statistical test (Table 3). Treatment failure as a tolerating index was evaluated and venlafaxine ER had a significantly higher treatment failure rate than citalopram (36% vs. 8%, P value: 0.01). None of the patients developed significant blood pressure change during the study (P > 0.05).
| 37.12 ± 10.94 | 35.60 ± 8.72 | 36.23 ± 9.58 | 0.63 | |
| 0.17 | ||||
| Male | 4 (25) | 2 (9) | 6 (15) | |
| Female | 12 (75) | 21 (91) | 33 (85) | |
| 21.37 ± 21.46 | 25.78 ± 23.26 | 23.97 ± 22.36 | 0.55 |
a Data are presented as No. (%) or Mean ± SD.
b Extended release.
| Drug Groups/P Value | Pretreatment HDRS Score | Post treatment HDRS Score | P Value |
|---|---|---|---|
| 19.87 ± 4.61 | 6.93 ± 4.13 | < 0.001 | |
| 17.63 ± 4.09 | 5.19 ± 2.57 | < 0.001 | |
| 0.11 | 0.11 |
a Data are presented as Mean ± SD.
b Hamilton depression rating scale.
c Extended release.
| Groups | Time Interval | P Value | ||||
|---|---|---|---|---|---|---|
| 19.88 ± 4.61 | 14.47 ± 4.48 | 11 ± 4.07 | 8.38 ± 3.7 | 6.94 ± 4.13 | < 0.001 | |
| 17.63 ± 4.09 | 12.87 ± 3.78 | 9.97 ± 3.58 | 6.48 ± 3.28 | 5.2 ± 2.57 | < 0.001 | |
| 0.155 | 0.193 | 0.548 | 0.074 | 0.217 | - | |
a Data are presented as Mean ± SD.
b Extended release.
5. Discussion
This study was conducted to compare the efficacy and tolerability of venlafaxine ER with those of citalopram in patients with major depression. Similar to several other placebo-controlled studies, the present study also demonstrated that both venlafaxine and citalopram are effective in the treatment of patients with major depression (6). Moreover, based on the insignificant difference between post intervention HDRS score in citalopram and venlafaxine treatment groups, it seems that these two medications may have equal efficacy in the treatment of major depression.
A previous study by Allard et al. confirms this finding. They performed a 6-month double-blind randomized clinical trial, and compared the efficacy and tolerability of venlafaxine ER with citalopram in geriatric outpatients. They concluded that venlafaxine ER induced beneficial effects of similar magnitude as those of citalopram (16). When two medications have similar efficacy, their other characteristics play more important roles in the process of the drug of choice selection for the patient. Toleration of a drug is among the most important characteristics that significantly affects the course of the treatment, and it will be more prominent when the long-term use of the medication is mandatory (17-19). The present study showed that treatment of major depression with venlafaxine caused a significantly higher rate of severe adverse effects, especially nausea and vomiting and it was the main cause for the drop out in the venlafaxine treatment group. This implies that citalopram could be a safer antidepressant for patients suffering from major depression. Allard et al. also found that citalopram was associated with a significantly lower rate of severe side-effects for patients compared to venlafaxine ER (16). All venlafaxine-treated patients who discontinued the treatment developed nausea and/or vomiting during the first 2 weeks of the treatment and none of the patients developed nausea or vomiting later, and it means that these kinds of side-effects are early onset type. Diaz-Martinez et al. who compared venlafaxine with fluoxetine also found similar finding and reported that nausea occurred most often during the first 1 or 2 weeks of treatment with venlafaxine (20).
We should declare that one of the major limitations of this study is the relatively small sample size, especially in the venlafaxine group, which had unpredictable dropout rate. The lack of a placebo group is among other limitations of this study that is plausible considering ethical issues.
Given almost the equal efficacy of citalopram and venlafaxine in the treatment of major depression and the higher rate of compliance for using citalopram, it would be better to consider citalopram as the first line of treatment in approach to the case of major depression. To improve the venlafaxine tolerance, more gradual titrates of the dose could be helpful that need to be found out in a clinical trial study. Finally, considering the high rate of dropout in the venlafaxine group, further studies with larger sample size are recommended to achieve more accurate results.