1. Background
With clients on methadone maintenance treatment (MMT), it is not uncommon for physicians to face situations that demand discontinuation of methadone. Those conditions might be clinical complications, environmental, or personal reasons, including cardiac complications (1), long-term side effects of methadone (2-6), movement of the client to geographical areas where methadone would not be available or even illegal, and the client’s wish to discontinue methadone for other reasons such as employment requirements. Tapering of methadone, however, puts some clients at risk of experiencing withdrawal symptoms and consequent lapse (7, 8). For discontinuation of methadone, therefore, while minimizing withdrawal symptoms, one should consider preventing negative consequences of drug termination such as sleep disorders, sexual dysfunction, overweight, depression, and anxiety (2, 3).
Brain circuits involved in stress management, learning, and social behavior closely interact with oxytocin mechanisms (9). Oxytocin has effectively been tried in substance use prevention (10, 11) and treatment (12-16). The anxiolytic and antidepressant effects of oxytocin may explain its effect in preventing the development of withdrawal symptoms upon abstinence (14). Interestingly, downgrading of endogenous oxytocin in response to substance use results in tolerance, drug-seeking behavior, and indifference to social rewards (14). As sub-stance dependence is explained as pathological learning (9, 10), oxytocin’s interference in neurobiological mechanisms of memory and learning may explain its role in substance use treatment (9, 17). Regarding the safety of intranasal administration of oxytocin, it appears to have no considerable side effects or adverse outcomes (18).
Oxytocin also sets a preference for memory of social reward over non-social rewards (19). This may explain the role of oxytocin in the replacement of substance-using behavior with social interactions (14). The alcoholics anonymous (AA) and narcotics anonymous (NA) meetings and interactions that help individuals keep sober (20, 21) may be examples of social-induced oxytocin release in clients (22). Oxytocin has also been shown to play a role in the treatment of anxiety, depression, sleep disorders, obesity, and sexual dysfunction (23-29). The depression-reducing impact of self-help groups (30) may also be linked to oxytocin effects (22).
Similar to some other cultures (31), in a traditional grieving ritual in Iran participants have been observed to perform self-mutilating practices without experiencing any pain. As in both examples, the core element is highly emotional group interactions, oxytocin may play a role in relieving pain (32-35). In some instances, the same group ritual has been used for abrupt dis-continuation of substances in dependent individuals and, interestingly, has averted withdrawal symptoms (First author observation).
2. Objectives
The present study aimed to evaluate and compare the role of oxytocin and group interactions, combined and independently, in abrupt discontinuation of methadone in MMT cases, where there had been a reasonable clinical judgment to cease medication.
3. Methods
3.1. Study Design
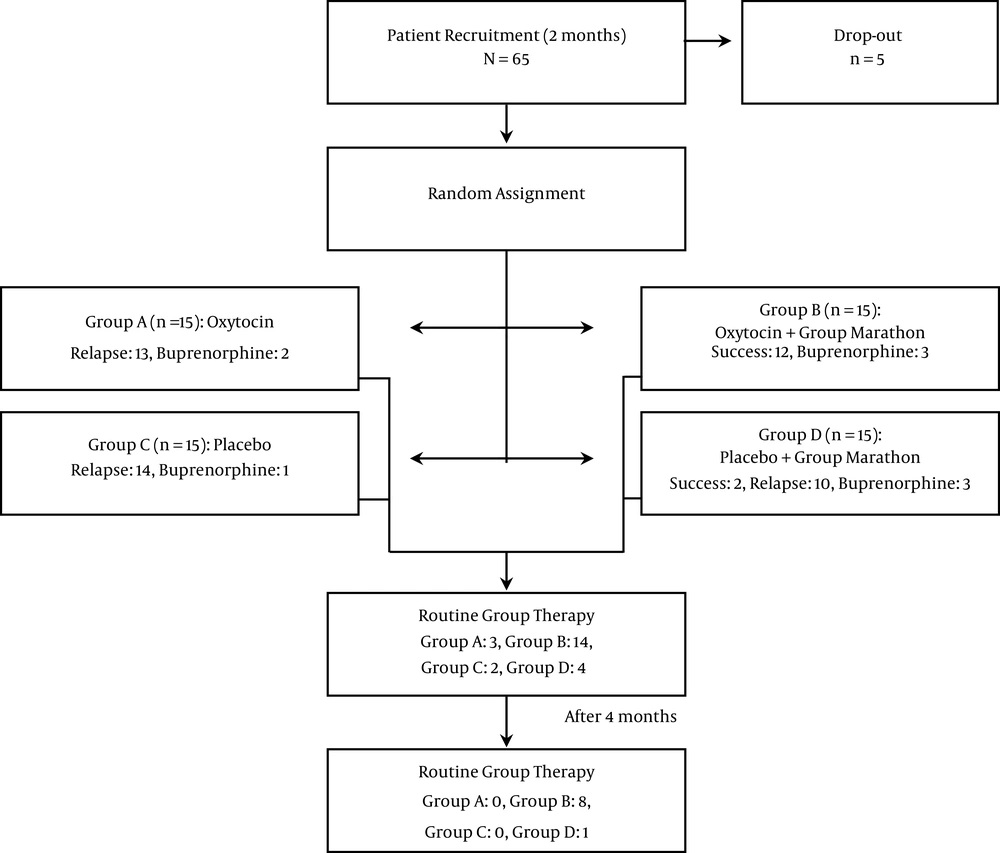
This study was a randomized, double-blind controlled clinical trial. Client recruitment took place in a 2-month period. Clients were randomly assigned to four groups by a psychologist who was not involved in the rest of the study (Figure 1). For participants as-signed to interventions, including group activities as soon as the first group of seven clients was assigned to each group, the group activity was started. In the meantime, recruitment and assignment for the second group of eight clients continued and upon accomplishment, the second round of group activity was undertaken.
3.2. Study Participants
Participants were longtime male MMT clients who had already negotiated a clinical indication to quit MMT with their therapist, but tapering their medication had not been successful. In most instances, the participants who were on high doses of methadone, while trying to taper off their medication had been incapacitated at lower doses and unable to complete the process. Having been on MMT (15 - 100 mg/d) for longer than six months with no positive urine tests for substances during the past three months, aged 18 - 55, insisting on discontinuing medication, and providing written informed consent were the inclusion criteria of the study. The exclusion criteria were acute cardiac problems, epilepsy, severe depression, suicidal ideation, and psychosis. The study did not provide incentives, but the whole course of the study was free of charge.
3.3. Data Gathering and Tools
Withdrawal symptoms were evaluated as a Likert scale on a self-report basis on days 2, 3, and 4 after quitting methadone. We tested the internal consistency of our withdrawal symptoms-monitoring questionnaire based on its results. The questionnaire’s internal consistency Cronbach’s alpha was 0.949. Furthermore, as Kaiser-Meyer-Olkin (KMO) and Bartlett’s tests were 0.88 and 0.000, respectively, we concluded that our questionnaire had acceptable internal consistency, sampling adequacy and at least, three components of the questionnaire were suitable for factor analysis. Also, performing varimax rotation method three components of craving, insomnia, and yawning had scores higher than 1, indicating an acceptable construct validity of the questionnaire. Throughout the 4 months of the study, a rapid urine test for morphine, methadone, and methamphetamine was performed weekly. Beck depression (36) and anxiety (37) inventories were used for scoring the participants’ status on days 1 and 5 of the study. Serum oxytocin was measured on days 1 and 4.
3.4. Interventions
The participants refrained from taking methadone 24 hours prior to joining groups A-D (group A: Oxytocin, no marathon group; group B: Oxytocin combined with marathon group; group C: placebo, no marathon group; group D: placebo combined with marathon group), according to their assignment. A single puff of nasal oxytocin spray (OxyPure Oxytocin Nasal Spray-12IU, PherLuv LLC) was used in each nostril 4 times a day on days 2 to 4. From day 5 to 11, oxytocin was administered only once a day in the evenings. Normal saline-filled in the same bottles of oxytocin was used as a placebo. The prescriber of the nasal sprays and clients were both blind to the content of the bottles. While clients assigned to the groups A and C received their medication at the MMT office, clients assigned to groups B and D spent a marathon group activity in a residential facility on days 2 - 4. Group activity during this period followed a structured algorithm with emotionally enriched activities. Group activities comprised of both mental activities, including playing and listening to music, watching video clips with emotional content, relaxation, guided imagery, guided thought blocking, and group discussions and physical activities, including dancing, massage, and playing active games. During days 2 to 11, for participants experiencing mild sleep disturbances clonidine 0.2 mg q.h.s and, in more severe cases, gabapentin 300 mg q.h.s were pre-scribed. For mild to moderate pain ibuprofen 400 - 800 mg/d was used. For cases experiencing moderate to severe withdrawal symptoms, a return to methadone was offered and the participants returning to over 50% of the dose prior to quitting were indicated as relapse. However, after day 11, the participants experiencing severe withdrawal symptoms were offered to be treated by buprenorphine instead of methadone. Those cases were also regarded as relapse. For participants who were taking other non-opioid prescription medications, no interference in their treatment was made. All participants, irrespective of their performance in the study, were offered to take part in regular twice a week group meetings for 4 months.
3.5. Statistical Analysis
We used IBM SPSS Statistics 23 software for statistical analysis. Also, we performed Kolmogorov-Smirnov and Levene tests for examination of normality and homogeneity of data. We performed univariate and bivariate general linear model for the analysis of variance to compare the averages of variables.
4. Results
Based on the characteristics of the participants (Table 1) the average age of participants was 37.7, and was on MMT for 3 years with an average dose of 42.3 mg/d. As mentioned earlier, the average methadone dose only reflects the dose prior to this study. But the actual dose that the participants had taken during their course of MMT generally were much higher. Between group ANOVA showed that regarding the characteristics, the four groups were similar to each other.
| Characteristic | Total | Group A | Group B | Group C | Group D | Significance (ANOVA |
|---|---|---|---|---|---|---|
| Age, y | 37.7 ± 8.6 | 37.9 ± 9.9 | 37.3 ± 7.6 | 37.3 ± 7.9 | 38.1 ± 9.7 | 0.991 |
| Time on MMT, y | 3 ± 1.6 | 2.9 ± 1.2 | 3.5 ± 1.8 | 2.9 ± 1.6 | 2.7 ± 1.9 | 0.732 |
| Dose of methadone, mg/db | 42.33 ± 21 | 39.7 ± 21 | 39.67 ± 20 | 42.7 ± 25 | 47.33 ± 19 | 0.608 |
aValues are expressed as mean ± SD.
bDaily dose of methadone at the beginning of the intervention.
We used a questionnaire with 18 items for the measurement of methadone withdrawal symptoms. The questionnaire’s internal consistency Cronbach’s alpha was 0.949. Furthermore, as Kaiser-Meyer-Olkin (KMO) and Bartlett’s tests were 0.88 and 0.000, respectively, we concluded that our questionnaire had acceptable internal consistency, sampling adequacy, and that at least three components of the questionnaire were suitable for factor analysis. Also, per-forming varimax rotation method three components of craving, insomnia, and yawning had scores higher than 1, indicating an acceptable construct validity of the questionnaire.
For days 2 - 4, the average severity of six symptoms of craving, muscle cramps, lack of appetite, perspiration, lethargy, and rhinorrhea had a normal distribution in all groups. Therefore, we analyzed those factors as parametric data compared to the other 12 factors that were analyzed as nonparametric data. As the test of homogeneity of variances showed a normal distribution for five of withdrawal symptoms, we performed ANOVA for intergroup differences of those symptoms, which showed a significant difference between groups for withdrawal symptoms of craving, muscle cramps, lack of appetite, rhinorrhea, and lethargy. We, therefore, performed Fisher’s least significant difference test between groups for those symptoms. The result showed that the mean of withdrawal symptoms in Group B was significantly different compared to other groups (Table 2). For perspiration, despite a normal distribution in all groups, within groups’ variance was not equal, one-way ANOVA Tamhane’s t2 test showed a significant difference in the mean score of perspiration in Group B compared to the other groups.
| Dependent Variable | Values | Sig | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Craving | ||||
| Group A | ||||
| Group B | 3.71333b ± 0.64452 | 0.000 | 2.4222 | 5.0045 |
| Group C | -0.12000 ± 0.64452 | 0.853 | -1.4111 | 1.1711 |
| Group D | 1.16200 ± 0.64452 | 0.077 | -0.1291 | 2.4531 |
| Group B | ||||
| Group A | -3.71333b ± 0.64452 | 0.000 | -5.0045 | -2.4222 |
| Group C | -3.83333b ± 0.64452 | 0.000 | -5.1245 | -2.5422 |
| Group D | -2.55133b ± 0.64452 | 0.000 | -3.8425 | -1.2602 |
| Group C | ||||
| Group A | 0.12000 ± 0.64452 | 0.853 | -1.1711 | 1.4111 |
| Group B | 3.83333b ± 0.64452 | 0.000 | 2.5422 | 5.1245 |
| Group D | 1.28200 ± 0.64452 | 0.052 | -0.0091 | 2.5731 |
| Group D | ||||
| Group A | -1.16200 ± 0.64452 | 0.077 | -2.4531 | 0.1291 |
| Group B | 2.55133b ± 0.64452 | 0.000 | 1.2602 | 3.8425 |
| Group C | -1.28200 ± 0.64452 | 0.052 | -2.5731 | 0.0091 |
| Muscle cramps | ||||
| Group A | ||||
| Group B | 2.29933b ± 0.69284 | 0.002 | 0.9114 | 3.6873 |
| Group C | 0.26933 ± 0.69284 | 0.699 | -1.1186 | 1.6573 |
| Group D | 0.41000 ± 0.69284 | 0.556 | -0.9779 | 1.7979 |
| Group B | ||||
| Group A | -2.29933b ± 0.69284 | 0.002 | -3.6873 | -0.9114 |
| Group C | -2.03000b ± 0.69284 | 0.005 | -3.4179 | -0.6421 |
| Group D | -1.88933b ± 0.69284 | 0.009 | -3.2773 | -0.5014 |
| Group C | ||||
| Group A | -0.26933 ± 0.69284 | 0.699 | -1.6573 | 1.1186 |
| Group B | 2.03000b ± 0.69284 | 0.005 | 0.6421 | 3.4179 |
| Group D | 0.14067 ± 0.69284 | 0.840 | -1.2473 | 1.5286 |
| Group D | ||||
| Group A | -0.41000 ± 0.69284 | 0.556 | -1.7979 | 0.9779 |
| Group B | 1.88933b ± 0.69284 | 0.009 | 0.5014 | 3.2773 |
| Group C | -0.14067 ± 0.69284 | 0.840 | -1.5286 | 1.2473 |
| Lack of appetite | ||||
| Group A | ||||
| Group B | 2.28933b ± 0.75139 | 0.004 | 0.7841 | 3.7945 |
| Group C | -0.69533 ± 0.75139 | 0.359 | -2.2005 | 0.8099 |
| Group D | 0.17800 ± 0.75139 | 0.814 | -1.3272 | 1.6832 |
| Group B | ||||
| Group A | -2.28933b ± 0.75139 | 0.004 | -3.7945 | -0.7841 |
| Group C | -2.98467b ± 0.75139 | 0.000 | -4.4899 | -1.4795 |
| Group D | -2.11133b ± 0.75139 | 0.007 | -3.6165 | -0.6061 |
| Group C | ||||
| Group A | 0.69533 ± 0.75139 | 0.359 | -0.8099 | 2.2005 |
| Group B | 2.98467b ± 0.75139 | 0.000 | 1.4795 | 4.4899 |
| Group D | 0.87333 ± 0.75139 | 0.250 | -0.6319 | 2.3785 |
| Group D | ||||
| Group A | -0.17800 ± 0.75139 | 0.814 | -1.6832 | 1.3272 |
| Group B | 2.11133b ± 0.75139 | 0.007 | 0.6061 | 3.6165 |
| Group C | -0.87333 ± 0.75139 | 0.250 | -2.3785 | 0.6319 |
| Rhinorrhea | ||||
| Group A | ||||
| Group B | 2.87133b ± 0.58488 | 0.000 | 1.6997 | 4.0430 |
| Group C | -0.07667 ± 0.58488 | 0.896 | -1.2483 | 1.0950 |
| Group D | 0.98600 ± 0.58488 | 0.097 | -0.1856 | 2.1576 |
| Group B | ||||
| Group A | -2.87133b ± 0.58488 | 0.000 | -4.0430 | -1.6997 |
| Group C | -2.94800b ± 0.58488 | 0.000 | -4.1196 | -1.7764 |
| Group D | -1.88533b ± 0.58488 | 0.002 | -3.0570 | -0.7137 |
| Group C | ||||
| Group A | 0.07667 ± 0.58488 | 0.896 | -1.0950 | 1.2483 |
| Group B | 2.94800b ± 0.58488 | 0.000 | 1.7764 | 4.1196 |
| Group D | 1.06267 ± 0.58488 | 0.075 | -0.1090 | 2.2343 |
| Group D | ||||
| Group A | -0.98600 ± 0.58488 | 0.097 | -2.1576 | 0.1856 |
| Group B | 1.88533b ± 0.58488 | 0.002 | 0.7137 | 3.0570 |
| Group C | -1.06267 ± 0.58488 | 0.075 | -2.2343 | 0.1090 |
| Lethargy | ||||
| Group A | ||||
| Group B | 2.62733b ±0.65995 | 0.000 | 1.3053 | 3.9494 |
| Group C | -0.30800 ± 0.65995 | 0.643 | -1.6300 | 1.0140 |
| Group D | -0.42000 ± 0.65995 | 0.527 | -1.7420 | 0.9020 |
| Group B | ||||
| Group A | -2.62733b ± 0.65995 | 0.000 | -3.9494 | -1.3053 |
| Group C | -2.93533b ± 0.65995 | 0.000 | -4.2574 | -1.6133 |
| Group D | -3.04733b ± 0.65995 | 0.000 | -4.3694 | -1.7253 |
| Group C | ||||
| Group A | 0.30800 ± 0.65995 | 0.643 | -1.0140 | 1.6300 |
| Group B | 2.93533b ± 0.65995 | 0.000 | 1.6133 | 4.2574 |
| Group D | -0.11200 ± 0.65995 | 0.866 | -1.4340 | 1.2100 |
| Group D | ||||
| Group A | 0.42000 ± 0.65995 | 0.527 | -0.9020 | 1.7420 |
| Group B | 3.04733b ± 0.65995 | 0.000 | 1.7253 | 4.3694 |
| Group C | 0.11200 ± 0.65995 | 0.866 | -1.2100 | 1.4340 |
aValues are expressed as mean ± SD.
bSignificant at ≤ 0.05
Interestingly, participants in group B with methadone doses of higher than 40 mg/d experienced the same withdrawal symptoms compared to the participants with lower doses of methadone. In order to compare the effect of group activity versus medication, we performed univariate analysis of variance for two dependent variables of craving and muscle cramps (Table 3). In both instances of group activity alone and group activity augmented by oxytocin, a significant association with the mean score of the two withdrawal symptoms of craving and muscle cramps was observed compared to oxytocin alone. Moreover, Group B participants showed significantly more pertinent continued abstinence in the four-month follow-up. Oxytocin and group activity alone or group activity augmented by oxytocin all showed a significant effect on reducing craving for methadone and muscle cramps. However, the existence of group activity as an intervention showed the highest power, when measuring their effect size (Table 3). On the basis of Kolmogorov-Smirnov, Levene, and Shapiro-Wilk tests, serum oxytocin level before and after the use of nasal spray showed a normal distribution but not with equal variance in all groups. ANOVA did not show a significant difference between and within groups in serum oxytocin following nasal spray.
| Type III Sum of Squares | df | Mean Square | F | Partial Eta Squared | Sig | |
|---|---|---|---|---|---|---|
| Craving | ||||||
| Corrected model | 142.503a | 3 | 47.501 | 15.246 | 0.450 | 0.000 |
| Intercept | 1448.745 | 1 | 1448.745 | 465.001 | 0.893 | 0.000 |
| Oxytocin | 26.760 | 1 | 26.760 | 8.589 | 0.133 | 0.005 |
| Group activity | 93.575 | 1 | 93.575 | 30.035 | 0.349 | 0.000 |
| Oxytocin + group activity | 22.168 | 1 | 22.168 | 7.115 | 0.113 | 0.010 |
| Error | 174.472 | 56 | 3.116 | |||
| Total | 1765.721 | 60 | ||||
| Corrected total | 316.975 | 59 | ||||
| Muscle cramps | ||||||
| Corrected model | 49.642b | 3 | 16.547 | 4.596 | 0.198 | 0.006 |
| Intercept | 626.232 | 1 | 626.232 | 173.942 | 0.756 | 0.000 |
| Oxytocin | 9.841 | 1 | 9.841 | 2.734 | 0.047 | 0.104 |
| Group activity | 22.326 | 1 | 22.326 | 6.201 | 0.100 | 0.016 |
| Oxytocin + group activity | 17.474 | 1 | 17.474 | 4.854 | 0.080 | 0.032 |
| Error | 201.613 | 56 | 3.600 | |||
| Total | 877.487 | 60 | ||||
| Corrected total | 251.255 | 59 |
aR squared = 0.450 (adjusted R squared = 0.420)
bR squared = 0.198 (adjusted R squared = 0.155)
We examined the participants using Beck depression and anxiety inventories’ scores before the intervention and on day five. As within groups mean of scores did not show normal distribution, the Wilcoxon signed-rank test was performed (Table 4). Both depression and anxiety mean scores showed a significant decrease in the group B only. Additionally, Group A also experienced a significant reduction in their anxiety.
aBeck Depression Inventory score.
bBeck Anxiety Inventory score.
cBased on positive ranks.
dBased on negative ranks.
5. Discussion
Group activity may alter several endocrine and neurotransmitter mechanisms such as cortisol and endorphins (38). However, we designed a model to specifically focus on oxytocin. Despite previous studies that the administration of nasal oxytocin ameliorated opioid withdrawal symptoms in animals (14), in the current study, oxytocin alone was ineffective in controlling opioid withdrawal symptoms. Nevertheless, we were able to show that a combination of oxytocin and group activity was effective in reducing craving, muscle cramps, lack of appetite, rhinorrhea, lethargy, perspiration, yawning, restlessness, insomnia, irritability, lacrimation, hot flashes, and boredom (Table 2). Similar to the previous evidence (39, 40), in the current study, the dose of methadone was not associated with the severity of withdrawal symptoms. Therefore, we conclude that in clinically justified situations that there is an indication for discontinuation of methadone, a combined package of oxytocin and group activity evades methadone withdrawal symptoms irrespective of methadone dose.
In our four-month follow-up, we found that in participants receiving combination of oxytocin and group activity that had fewer withdrawal symptoms, continued abstinence was more common. This pattern resembles the same effect of narcotics self-help groups on abstinence (41, 42), where regular attendance results in higher abstinence. Given that the participants who received the combined package of oxytocin and group activity were the same group with higher participation in regular group sessions, the four-month follow-up of the study further revealed the impact of the package on relapse prevention (Table 3).
The finding that serum oxytocin level showed no difference within and between groups (Table 3), reconfirms the evidence that serum oxytocin level is not associated with oxytocin activity in the brain (14). However, the fact that anxiety and depression were significantly ameliorated upon administration of oxytocin combined with group activities, along with the observation of a higher tendency to continue participation in routine group therapy sessions might indicate that, similar to animal studies (9, 14, 27), an elevated brain oxytocin activity would not only ameliorate opioid withdrawal symptoms but also could result in better abstinence records in the long run. The significance of this finding is that lower depression and anxiety scores were recorded just after 72 hours of marathon group activity, reflecting the acute effect of oxytocin (29, 43-46). It appears that a larger sample of participants, extended periods of group activities, and longer follow-up periods might further improve the findings of this study. It has been argued that in higher doses, oxytocin will result in rebound secretion of vasopressin and blocked effect of oxytocin (9, 47). As in our study, the participants received relatively high doses of more than 100 units of oxytocin, we suggest that marathon group activity reversed this effect.
All male participants could be considered a limitation of the study. Also, a larger number of participants assigned to study groups could result in more accurate results. As the participants in the current study were long-term opioid users, duplicate studies on individuals who use other substances could further reveal whether the oxytocin effect is inclined toward the behavioral aspect of substance use or its pharmacological effects on specific substances. We, therefore, would like to suggest studying the findings of this study in other disorders where oxytocin is found to play a role such as depression (27, 44), anxiety disorders (26, 27, 29), attention deficit hyperactivity disorder (27, 48), autism (26, 27), and eating disorders (25-27).
5.1. Conclusions
The findings of this study reveal that in the process of abstaining from opioids, withdrawal symptoms may be avoided by oxytocin. However, exogenous oxytocin alone may not be the pertinent method of administration. Rather, it appears participation in group activities with highly emotional themes resulting in endogenous oxytocin release not only controls withdrawal symptoms but also results in continued abstinence.