1. Background
Autism spectrum disorders (ASD) are characterized by the 2 main areas of disturbance in social communication and restricted, repetitive patterns of interest, and behaviors (1). The global prevalence of autism spectrum disorders is 62 in 10000 children (2). Various factors suggested a biological origin of ASD in addition to the role of genetic factors in this disorder (3, 4). Numerous groups of medications, including atypical antipsychotics, have been used for treatment of associated behavioral problems such as aggression and self-injury (5, 6). Many patients with ASD also exhibit the symptoms of attention deficit hyperactivity disorder (ADHD). In addition, 1/4 of ASD children receive stimulants for ADHD treatment (7). Reports express an increased prescription of methylphenidate and Atomoxetine in autistic patients (3).
The latest literature reviews mentioned the effect of Atomoxetine on ADHD symptoms of ASD patients. The improvement of such symptoms, by using Atomoxetine, was reported in most studies. Nevertheless, therapeutic response and side effects of Atomoxetine are contradictory in most studies. Furthermore, there are few clinical trials in this area (7).
For example, several studies have reported that Atomoxetine improves ADHD symptoms. Also, the side effect profile is well tolerated versus other treatments (8). The safety and effectiveness of Atomoxetine was proved in patients with ASD for social withdrawal and ADHD symptoms (9). The study of Harfterkamp et al., (10) revealed no effectiveness of Atomoxetine on social performance, except a little impact on stereotyped behaviors.
As aforementioned, results of different studies showed the inconsistency in the effectiveness of atomoxetine. The authors investigated the role of Atomoxetine in the treatment of autistic feature in ASD through a double blind, Placebo controlled trial. The findings of this study can be used to relieve some symptoms of autism. Also, other aspect of Atomoxetine such as potential side effects could be determined.
2. Objectives
The purpose of this study was the assessment of Atomoxetine effectiveness and tolerability in the treatment of autistic features in patients with ASD.
3. Patients and Methods
The present study is an 8 week, randomized Placebo-controlled clinical trial conducted at the outpatient clinic of Ibne-Sina hospital and Dr sheikh hospital affiliated to the Mashhad Medical University in Iran, between August 2015 to September 2016. The participants were evaluated by the childhood autism rating scale (CARS), clinical global impression (CGI), and side effect checklist. The CARS is one of the most widely used diagnostic tools for ASD and as the primary outcome measure too (11-15). This test is valid and reliable across time and raters (16). Also, good reliability and validity of CARS are reported in an unpublished study in Iran (17). The CARS included 15 items, each rated by 7-point Likert scale (1 - 4 with ½ points). The total score of CARS ranges from 15 - 60. The increment of the score shows higher probability or severity of ASD. The score of 15 - 30 are considered not autistic; 30.5 - 36 mildly or moderately autistic; 36.5 - 60 severely autistic.
The CGI is the brief, clinician administered, well-established research rating tool applicable to all psychiatric disorders and can track clinical progress across time (18). Our assessment showed good face and content validity of the Persian translation of CGI. It included 3 items (severity of illness, global improvement, efficacy index). The 1st and 2nd items are used in our study. These 2 items of CGI suggest a practical measurement tool and can simply be used by a clinician in a busy clinical setting. The items of clinical global impression improvement scale (CGI-I) are rated by clinical judgment from 1 to 7 degrees. The score of 1 are considered very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; 7, very much worse. The score of severity of illness are rated from 0 to 6 degrees too. The score of 1, are considered not at all ill; 2, borderline mentally ill; 3, mildly ill; 4, moderately ill; 5, markedly ill; 6, severely ill; 7, among the most extremely ill patients (19, 20).
3.1. Participants
A total of 44 children and adolescents with ASD, aged from 6 to 17, enrolled in the study. All of the participants met the DSM-5 criteria for autism spectrum disorders, according to the evaluation of the child and adolescent psychiatrist (21). The patients aren’t a new case of ASD. All of the subjects previously received adequate dose of risperidone for at least 6 months. The exclusion criteria were concomitant ADHD (ADHD-RS > 15) and other psychiatric disorders (for example, substance use disorders, tic disorders, psychotic disorders and so on), any medical conditions, severe mental retardation (IQ < 50), and using any psychotropic medication except risperidone.
3.2. Study Design
All patients were assessed in terms of psychiatric interview, general medical condition, and physical examination. The diagnosis of ASD was proved by the child and adolescent psychiatrists based on semi-structured interview with the parents according to DSM-5 criteria. All patients were using risperidone at the beginning of the study with a range dose of 1 - 4 mg. Patients were randomly assigned to receive Atomoxetine augmentation or Placebo augmentation in 1:1 ratio. Atomoxetine was initiated at 0.5 mg/kg/day. Child and adolescent psychiatrist gradually titrated dose of Atomoxetine up to 1.2 mg/kg/day based on clinical effectiveness and the patient’s tolerability. Atomoxetine was divided in 2 doses in the morning and early afternoon. Atomoxetine is used under the brand name of Stramox. Placebo was entirely similar to Atomoxetine. It was dispensed by the investigational drug pharmacist. Throughout the study, questionnaires were completed by the trained resident of psychiatry. The minimum score of 30 on the CARS was required for enrollment into the study. Patients were evaluated at baseline (T0) and 4 weeks (T1) and 8 weeks (T2) after the medication administrated. The side effects of medications were appraised with the side effect checklist every 4 weeks.
3.3. Ethical Considerations
The study was conducted after registration in the Iranian registry of clinical trials (IRCT) center and approved by the University ethical committee (Ethics code: IR.MUMS.REC.1394.95). Then, informed consent was obtained from parents of all patients. In all steps of research, medical confidentiality and privacy were respected. The clinical trial registration number is: IRCT2016022826802N1.
3.4. Statistical Analysis
Mixed ANOVA -group as between group and time as within the group was conducted. P ≤ 0.05 were considered for statistical significance.
4. Results
A total of 44 outpatient children and adolescent with ASD (36 male and 8 female), aged 6 to 17 (mean ± SD, 8.02 ± 2.27) enrolled in the present study. In total, 40 patients completed the trial. At the baseline of trial, according to CARS and SI subscale of CGI, 6 patients were mildly ill, 17 were moderately ill, 10 were markedly ill, and 7 were severely ill. Basic demographic characteristics such as gender, age, duration, and severity of illness did not significantly differ between 2 groups (Table 1).
| Variables | Atomoxetine Group | Placebo Group |
|---|---|---|
| Age, mean ± SD | 8.2 ± 2.2 | 7.8 ± 2.3 |
| Gender | ||
| Male | 16 | 18 |
| Female | 4 | 2 |
| Severity of illness | ||
| Mildly ill | 2 | 4 |
| Moderately ill | 8 | 9 |
| Markedly ill | 5 | 5 |
| Severely ill | 5 | 2 |
Demographic Data of Patients With Autism Spectrum Disorder
At the baseline of the study there were no significant differences between the 2 groups in total subscales of CARS and CGI scores. Mixed Anova, 1 between the group and 1 within the group were conducted for CARS and CGI and its subscales. In the subscales of Visual Response, Listening Response, Taste- Smell-touch response, and level and consistency of intellectual response, Mauchlys test is not significant for sphericity. Therefore, the results were interpreted according to sphericity assumption. Mauchly test showed significant differences in the other subscales of CARS and results were reported with Greenhause-Geisser correction.
4.1. Child Autism Rating Scale
The interactions (time and group of treatment) showed significant differences in the 7 subscales of CARS that included relationship to people (F = 1.734, df = 1.311, Df Error = 78, P = 0.0001), emotional response (F = 5.744, df = 1.358, Df Error = 51.621, P = 0.013), body use (F = 12.061, df = 1.515, Df Error = 54.532, P = 0.0001), listening response (F = 23.427, df = 2, Df Error = 76, P = 0.0001), fear and nervousness (F = 11.242, df = 1.653, Df Error = 62.821, P = 0.0001), nonverbal communication (F = 4.030, df = 1.464, Df Error = 55.621, P = 0.032), and activity level (F = 52.52, df = 1.709, Df Error = 64.958, P = 0.0001) (Table 2). There are no significant differences in the subscales of imitation, object use, adaptation to change, visual response, taste smell touch, verbal communication, level and consistency of intellectual response, and general impressions of CARS.
| Groups of Treatment | Atomoxetine Group | Placebo Group |
|---|---|---|
| Relationship to people | ||
| T0 | 2.95 (0.72) | 2.90 (0.52) |
| T1 | 2.72 (0.85) | 2.90 (0.52) |
| T2 | 2.55 (0.87) | 2.90 (0.52) |
| Emotional response | ||
| T0 | 2.62 (0.45) | 2.67 (0.76) |
| T1 | 2.45 (0.53) | 2.65 (0.77) |
| T2 | 2.40 (0.52) | 2.65 (0.77) |
| Body use | ||
| T0 | 3.12 (0.62) | 2.42 (0.84) |
| T1 | 2.95 (0.65) | 2.40 (0.82) |
| T2 | 2.72 (0.71) | 2.45 (0.75) |
| Listening response | ||
| T0 | 2.27 (0.52) | 2.20 (0.81) |
| T1 | 2.22 (0.49) | 2.20 (0.81) |
| T2 | 2.22 (0.49) | 2.20 (0.81) |
| Fear and nervousness | ||
| T0 | 3.00 (0.60) | 2.20 (0.78) |
| T1 | 2.85 (0.58) | 2.20 (0.78) |
| T2 | 2.62 (0.75) | 2.20 (0.78) |
| Nonverbal communication | ||
| T0 | 2.25 (0.59) | 2.27 (0.61) |
| T1 | 2.20 (0.54) | 2.27 (0.61) |
| T2 | 2.10 (0.44) | 2.27 (0.61) |
| Activity level | ||
| T0 | 3.20 (0.47) | 2.52 (0.71) |
| T1 | 2.72 (0.52) | 2.52 (0.71) |
| T2 | 2.27 (.54) | 2.50 (0.68) |
| Total score | ||
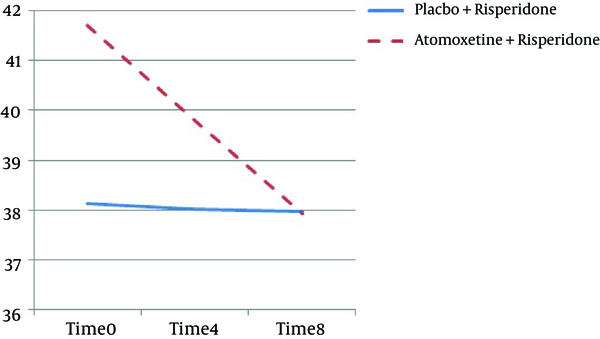
| T0 | 41.70 (6.55) | 38.12 (5.58) |
| T1 | 39.80 (6.55) | 38.02 (5.66) |
| T2 | 37.92 (6.73) | 37.97 (5.66) |
Also, the total score of CARS had significant difference between 2 groups (F = 33.77, df = 1.31, Df Error = 50.39, P = 0.001) (Figure 1).
4.2. CGI Rating Scale
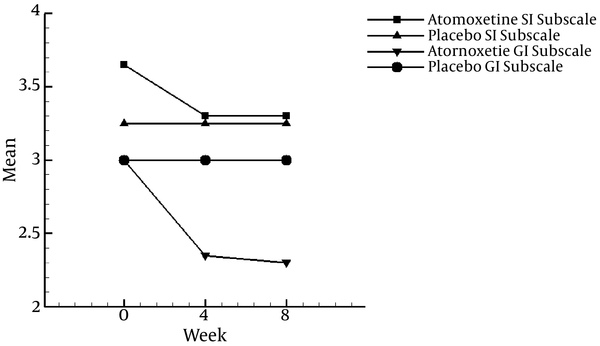
In the severity index (SI) of the CGI scale, Mauchlys test for sphericity is not significant. Therefore, the results were interpreted according to sphericity assumption, however, the general improvement (GI) of CGI was reported with Greenhause-Geisser correction. The interactions (time and group of treatment) were statistically meaningful in the both SI (F = 10.23, df = 1, Df Error = 76, P = 0.001,), and GI (F = 35.84, df = 1.33, Df Error = 55.57, P = 0.001) subscales (Figure 2 and Table 3)
| Groups of Treatment | Atomoxetine Group | Placebo Group |
|---|---|---|
| General improvement | ||
| T0 | 3.00 (0.00) | 3.00 (0.00) |
| T1 | 2.35 (0.48) | 3.00 (0.00) |
| T2 | 2.30 (0.47) | 3.00 (0.00) |
| Severity index | ||
| T0 | 3.65 (0.98) | 3.25 (0.91) |
| T1 | 3.30 (1.03) | 3.25 (0.91) |
| T2 | 3.30 (1.03) | 3.25 (0.91) |
Mean (SD) Scores for Clinical Global Impressiona
4.3. Adverse Effects
A total of 44 patients enrolled in the trial; 3 patients from the Atomoxetine group interrupted the trial, 1 of them desisted the trial, and 2 patients due to adverse effects (irritability and separation anxiety). In the Placebo group, treatment discontinuation didn’t happen due to the side effects, however, 1 patient refused the trial because of immigration. A total of 40 patients (%90) completed the trial (20 cases for each group). In the Atomoxetine group, 11 patients from 20 (%55) report side effects during the treatment. These complaints subsided without any intervention. The most common adverse effects were mood change, irritability, and GI disturbance.
In the Placebo group, 3 patients from 20 (15%) reported side effects. Adverse effects in this group were headache, GI disturbance, and decreased appetite; these complaints subside without any intervention too. It should be noted that mood change revealed significant differences between the 2 groups (Table 4).
| Variables | Frequency (%) | P Value |
|---|---|---|
| Anxiety | 1.00 | |
| Placebo | 0 (0.0) | |
| Atomoxetine | 1 (5) | |
| Neause and vomiting | 0.48 | |
| Placebo | 0 (0.0) | |
| Atomoxetine | 2 (10) | |
| Decreased appetite | 1.00 | |
| Placebo | 1 (5) | |
| Atomoxetine | 2 (10) | |
| Sleepy | 0.34 | |
| Placebo | 0 (0.0) | |
| Atomoxetine | 2 (10) | |
| Mood change | 0.02 | |
| Placebo | 0 (0.0) | |
| Atomoxetine | 6 (30) | |
| Irritability | 0.10 | |
| Placebo | 0 (0.00) | |
| Atomoxetine | 4 (20) | |
| GI disturbance | 0.34 | |
| Placebo | 1 (5) | |
| Atomoxetine | 4 (20) | |
| Headache | 1.00 | |
| Placebo | 1 (5) | |
| Atomoxetine | 1 (5) | |
| Tic | 1.00 | |
| Placebo | 0 (0.0) | |
| Atomoxetine | 1 (5) |
Adverse Effects in the Groups of Treatment
5. Discussion
Although different studies indicated various findings about the safety and efficacy of Atomoxetine, the results of the present study concluded that Atomoxetine is effective and relatively safe in the treatment of children and adolescent with ASD.
The past studies found various results compared to the present study. For example, Harfterkamp et al., (19) assessed 97 patients with ADHD and ASD, between the ages of 6-17 years, and were divided into 2 groups (Atomoxetine and placebo). The patients were evaluated through ADHD-RS, CGI-I, and conner’s teacher rating scale-revised- short form (CTRS-R:S). Their results showed that among subscales of CTRS-R, only hyperactivity was improved significantly in Atomoxetine group while there were no significant differences in CGI scores. The present study indicated the very marked difference between 2 groups in CGI scores. Harfterkamp et al., (19) side effects were reported in 81.3% of patients who received Atomoxetine and 65.3% of the Placebo group. According to the present study side effects were reported in 55% of patients who received Atomoxetine and 15% of the Placebo group. The differences between the results of 2 studies could be due to the excluding of ADHD patients from the present study.
Kilincaslan et al. (9) assessed the effect of Atomoxetine on ADHD and autistic symptoms among 37 ASD children through CGI, DSM-IV-based ADHD-rating scale (ADHD-RS), and Turkish version of the aberrant behavior checklist (ABC). The CGI scores were improved at the rate of 48.8% of patients. Also, the scores related to ADHD-RS and parent-reported ABC was decreased significantly. The present study also indicated the improvement in CGI score and activity level. Therefore, the results of the 2 studies indicated that Atomoxetine is a safe and effective medication among ASD patients.
In addition, Harfterkamp et al. (10) evaluated 97 patients with ASD and ADHD by ABC and children’s social behavior questionnaire (CSBQ). They found that Atomoxetine treatment led to significant improvement in ABC subscales and fear subscale of CSBQ had the same effect on CARS scores of the present study, however, Harfterkamp study indicated that Atomoxetine had no significant benefits on social functioning. Hence, the differences between the 2 studies regarding social functioning subscale could be due to different rating scale and exclusion of ADHD patients in the present study.
The results of Zeiner et al., (22) showed there were significant reductions in ADHD symptoms (P < 0.05) and Atomoxetine was tolerated in most cases as well. Their results were in partial agreement with the present study, although their study assessed only 1 gender (boys).
The results of a review article, which was published in 2014, indicated that Atomoxetine is an effective treatment for managing ADHD symptoms among patients with developmental disabilities such as ASD. Decreased appetite, nausea, and irritability were the most frequent adverse effects, which was the same as the present study (23). It should be noted that this review article did not evaluate autistic symptom.
Also, Handen et al., (8) evaluated 128 children with ASD and ADHD symptoms who were divided into 4 groups as Atomoxetine, Atomoxetine + Parent training, Placebo + Parent training, and Placebo. Instrument research were parent DSM-ADHD symptoms and home situation questionnaire (HSQ). The results showed that Atomoxetine and Parent Training (alone or combined) led to significant improvement in ADHD symptoms compared to Placebo, however, the effect of Atomoxetine is superior to arent training and Atomoxetine was tolerated by patients as well. The results of this study, in regards to the tolerability and activity level, were in partial agreement with our study, however, autistic symptom was not evaluated in Handen study.
Psychotropic medications (such as stimulants, α agonists, and Atomoxetine) are progressively used among children and adolescents with ASD, therefore, the widespread using of this treatment needs more attention and strong evidences, which support the effectiveness and safety of these medications in ASD patients (24).
5.1. Conclusions
The Atomoxetine add-on therapy showed significant improvement in 7 subscales and total score of CARS, global impression, and severity index of CGI (all P value ≤ 0.05). The study suggests that Atomoxetine may be a potential adjunctive treatment strategy for autism. As aforementioned, there are no significant differences in side events except mood change between 2 groups.
5.2. Limitation and Future Direction
The sample size was small and the duration of the current trial was very short. It is not clear whether the symptom will relapse after the discontinuation of Atomoxetine. It should be noted, psychometric properties of CGI are not evaluated in Iran.