1. Background
Major depressive disorder (MDD) is the most common indication for electroconvulsive therapy (ECT), the fastest and the most effective therapy for which is ECT. ECT is also effective for depressive and manic episodes in Bipolar I disorder (BID) (1). However, this technique is also associated with some cognitive side-effects, thereby arousing distress among patients (2). Various strategies have been adopted to decrease those adverse complications, some non-pharmacological of which include changing electrode placement, using ultra-brief (and brief) pulse waves to induce seizures, and reducing stimulation intensity (3). Moreover, pharmacological agents have also been used to alleviate ECT-induced cognitive impairments, some of which encompass adjunctive treatment with dexamethasone (4), vasopressin (5, 6), thyroid hormones (7), naloxone (8), calcium channel blockers (9), some medicinal plants (10), and memantine (11, 12) during ECT.
Concerning the role of the cholinergic system in memory and cognitive processes (13), some medications with cholinergic properties have also been examined for modulating post-ECT cognitive deficits. Some findings confirmed the use of rivastigmine and galantamine in decreasing the ECT-induced cognitive defects (14, 15). However, piracetam plays no remarkable role in alleviating the post-ECT cognitive impairments (16). Donepezil is an approved acetylcholinesterase inhibitor in treating mild to moderate Alzheimer’s diseases (17). To the best of our knowledge, few studies have evaluated the efficacy of donepezil in reducing the cognitive side-effects of ECT (18-21).
In a case report, Roa et al. (18) documented the beneficial effects of donepezil on cognitive performance in a patient using ECT. Prakash et al. (19) reported some practical outcomes with donepezil as an adjunct in relieving the ECT-induced cognitive impairments in a clinical trial. Their study encompassed 45 patients with different psychiatric disorders (namely schizophrenia, MDD, BID, delusional disorder, and unspecified psychosis). They assessed the participants’ cognitive performance after each ECT session. According to their findings, patients in the donepezil group experienced an accelerated post-ECT recovery of cognitive deficits significantly. Donepezil was administered before the first ECT session and continued three days after the last ECT. However, Shams-Alizadeh et al. (20) detected no effect of donepezil on the patients’ cognitive impairment. In their RCT, patients received 5 mg/d donepezil 24 hours before the study until the last ECT. The patients were cognitively assessed 24 hours before the first ECT and 48 hours after the last ECT session. According to Dutta et al. (21), donepezil did not improve cognitive outcomes after ECT.
2. Objectives
The contradictory outcomes may be caused by the differences in study designs, heterogenicity of patients' disorders, and sample size. Considering contradictory findings and few randomized clinical trials (RCT) in this field, the present study aimed to examine the effects of donepezil on the cognitive performance of ECT-treated patients with mood disorders.
3. Methods
3.1. Study Design
This double-blinded randomized placebo-controlled trial was conducted from July 2017 to March 2018 in the psychiatric wards of the Hajar Hospital affiliated to the Shahrekord University of Medical Sciences.
Considering the confidence level of 95%, 90% power, and the differences between the mean and standard deviation of cognitive scores reported in previous studies (MMSE score (primary outcome)) (12), the required sample size was 42 persons per group. In this regard, 15% of the estimated sample size was added to the samples to consider the attrition rate. The minimum acceptable sample size was calculated to be 96 persons. A total of 116 patients were screened regarding inclusion and exclusion criteria. Finally, 96 patients were randomly assigned to the donepezil and placebo groups. Statistical consulter generated allocation sequence using the random number generator software. To observe blindness, the psychiatrist who allocated the patients played no role in the treatment, and nurses providing the tablets to the patients were not informed of the groups. Moreover, investigating physician who assessed the participants using the concerned scales were not aware of the groups as well. Further, the patients were not aware of the nature of the assignment; however, they were notified about the study protocol, and all participants or their legal guardians provided written informed consent. This study was approved by the Ethics Committee of the Shahrekord University of Medical Sciences (code: IR. SKUMS.REC.1396.49) and registered in the Iranian Registry of Clinical Trials (www.irct.ir, identifier: IRCT20180623D40193N1). The study procedure conformed to the Helsinki declaration.
3.2. Participants
The 15-65-year-old patients with mood disorders, for whom ECT was adopted as a treatment. The patients meeting the following conditions were excluded from this study: cognitive disorders such as delirium and dementia, intellectual disabilities, substance abuse or dependency (except for caffeine and nicotine) during the study period, consuming any other memory-enhancing agents before and during the study, taking donepezil before the initiation of intervention, pregnancy, and breastfeeding, acute peptic ulcer, chronic obstructive pulmonary disease, any cardiac diseases prohibiting the use of acetylcholinesterase inhibitors such as bradyarrhythmias, any uncontrolled medical illness affecting the patient’s cognitive functions, discontinuation of ECT before the fourth session, and illiteracy.
Experienced psychiatrists diagnosed mood disorders and other comorbidities according to the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fifth edition (SCID-5). Moreover, all participants underwent a physical examination. Pre-study laboratory assessment included complete blood count with differential electrolytes and blood urea nitrogen, creatinine, and liver function test. The patients continued to take other drugs prescribed during the ECT sessions.
3.3. Intervention
In the intervention group, the patients received 5 mg/day of donepezil tablet (Raha pharmaceutics, Isfahan, Iran) from the first ECT session to the fourth week after the last ECT session. Similarly, the control group received a placebo with the same dose and treatment duration. Raha Pharmaceutical Company (Isfahan, Iran) manufactured the placebo tablets with same shape and color as donepezil tablets. The same series and preparation time were considered for all drugs. ECT was administered three times per week. The ECT sessions were determined to be 6 - 10 sessions based on the treating psychiatrist’s clinical judgment. All patients were prescribed atropine before anesthesia. Propofol (1 mg/kg) was used for general anesthesia with succinylcholine (1 mg/kg) as a muscle relaxant. We used the Thymatron DGX (Somatics Inc, Class 1 Type BF) to administer ECT, which was a square wave brief pulse device. The threshold of 15 seconds of motor seizure activity was considered for conducting stimulus energy. Seizure monitoring was performed using motor convulsive activity observation.
3.4. Outcome Measures
The patients were evaluated in terms of cognitive states 24 hours before the beginning of ECT, 2 hours after the fourth ECT session, and one month after the last ECT session using the reliable and validated Persian versions of Mini-Mental Status examination (MMSE) and Addenbrooke’s cognitive Examination-Revised (ACE-R) (22, 23). ACE-R is a valid brief cognitive scale incorporating five subscales (namely orientation, attention, verbal fluency, memory, language, and visuospatial). ACE-R is sensitive to early cognitive dysfunction (24).
In this study, the donepezil safety and tolerability were assessed during the intervention. The researcher asked patients to report any likely side-effects. The total scores of MMSE and ACE-R were selected as the primary and secondary outcomes, respectively. The primary and secondary outcomes were to assess whether donepezil in the study groups was associated with higher total scores of MMSE and ACE-R one month after the last ECT session.
3.5. Statistical Analysis
First, the normality of the collected data was checked using the Shapiro-Wilk test. Then the donepezil and placebo groups were compared regarding baseline demographic characteristics presented as mean ± SD or frequencies (percentage). An independent samples t-test was also run to compare quantitative variables, and chi-square analysis compared the qualitative data of the two groups by time points. General linear model (GLM) and repeated measures analysis of variance (ANOVA) investigated the time, treatment, and time–treatment interaction effects. The two groups and the three measurements during treatment were considered as a between-subjects factor (group) and within-subjects factor (time), respectively. The analysis were done for MMSE and ACE-R scores. There was a significant difference between the intervention and control groups regarding gender (P < 0.05). This variable was imported into the RM-ANOVA model as a covariate.
All statistical analysis was performed using the statistical package for social science software (SPSS) version 23 (Armonk, NY: IBM Corp). P < 0.05 was set as the level of significance.
4. Results
4.1. Patients’ Characteristics
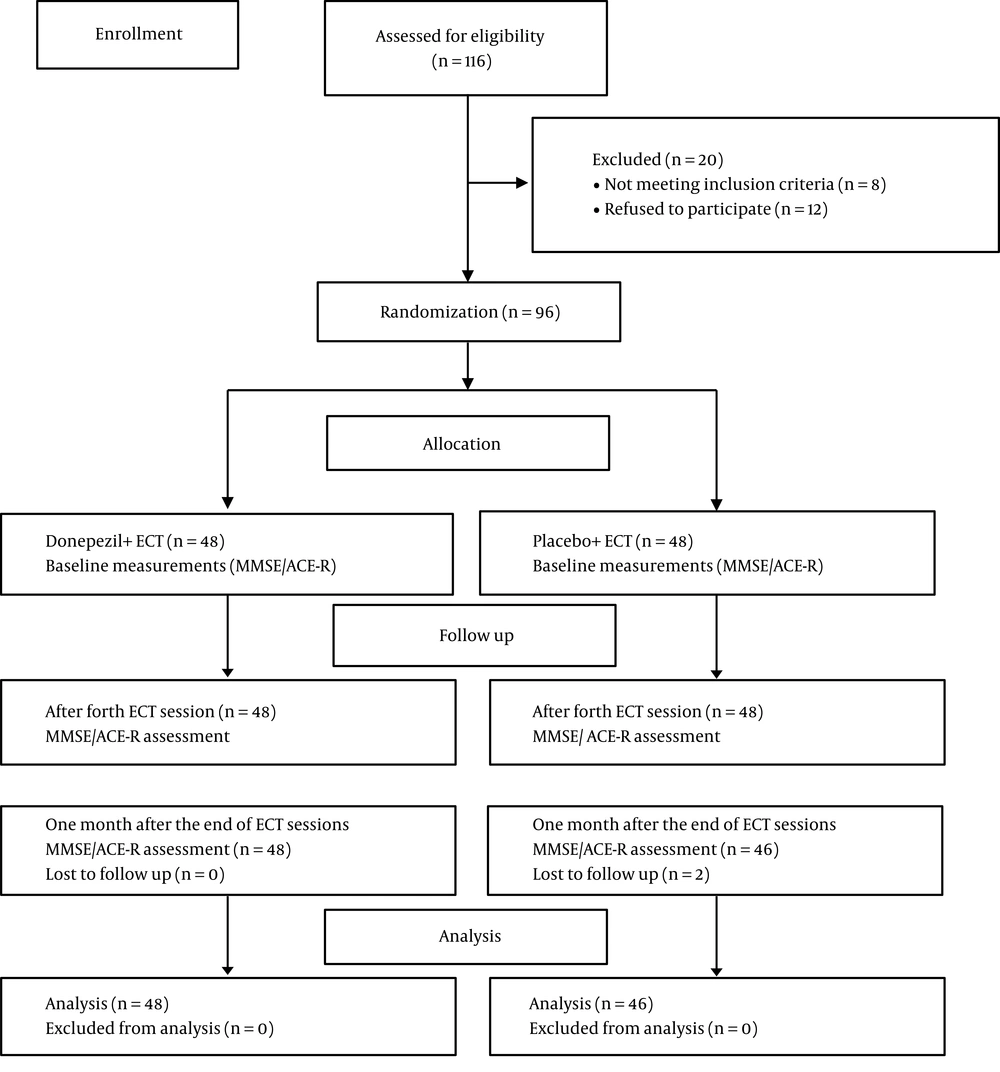
As shown in Figure 1, we assessed 116 patients to determine their eligibility, of whom 20 participants (eight patients not meeting inclusion criteria, and 12 persons refused to participate in the study) were excluded. In this regard, 48 patients were assigned to each group. Two patients from the placebo group lost the second follow-up time. Finally, 48 patients in the donepezil group and 46 patients in the placebo group were analyzed. Table 1 displays the demographic information of the patients in each group.
| Characteristics | Donepezil Group (N = 48) | Placebo Group (N = 46) | P-Value |
|---|---|---|---|
| Age, y | 45.81 ± 7.37 | 46.37 ± 4.67 | 0.64* |
| Gender | 0.023** | ||
| Female | 30 (62.5) | 18 (39.1) | |
| Male | 18 (37.5) | 28 (60.9) | |
| Education status | 0.31** | ||
| College certificate | 3 (6.2) | 5 (10.9) | |
| High school diploma | 18 (37.5) | 11 (23.9) | |
| Below high school diploma | 27 (56.2) | 30 (65.2) | |
| Patient diagnosis | 0.98* | ||
| Major depressive disorder | 26 (54.2) | 25 (54.3) | |
| Bipolar disorder | 22 (45.8) | 21 (45.8) | |
| Number of ECT sessions | 8 | 8 | - |
| Electrode placement | Bitemporal | Bitemporal | - |
| Stimulus, energy % | 34.7 ± 7.3 | 33.5 ± 5.7 | 0.51* |
| Seizure length*** | 34.45 ± 10.48 | 33.20 ± 8.49 | 0.69* |
| Baseline MMSE | 17.10 ± 6.01 | 15.97 ± 6.10 | 0.19 |
| Baseline total ACE-R | 60.18 ± 18.07 | 56.13 ± 21.16 | 0.26 |
aValues are expressed as No. (%) or mean ± SD.
b* Independent sample t-test; **, χ2-test; ***, seconds.
4.2. Efficacy Results
4.2.1. Primary Outcome (MMSE)
Running the RM-ANOVA, we first checked Mauchly’s test of sphericity. The P-value of this test was P < 0.001; hence, Greenhous-Geisser statistics was used to assess within-subjects effects. In this test, the treatment-time interaction was first checked, and the results were significant (F = 16.74, df = 1.67, P = < 0.001). It was also observed that the donepezil and placebo groups differed significantly regarding the ACE-R improvement at the beginning and the fourth week after the last ECT session as such different effects were not considered for time and treatment. However, the significant effects are described here.
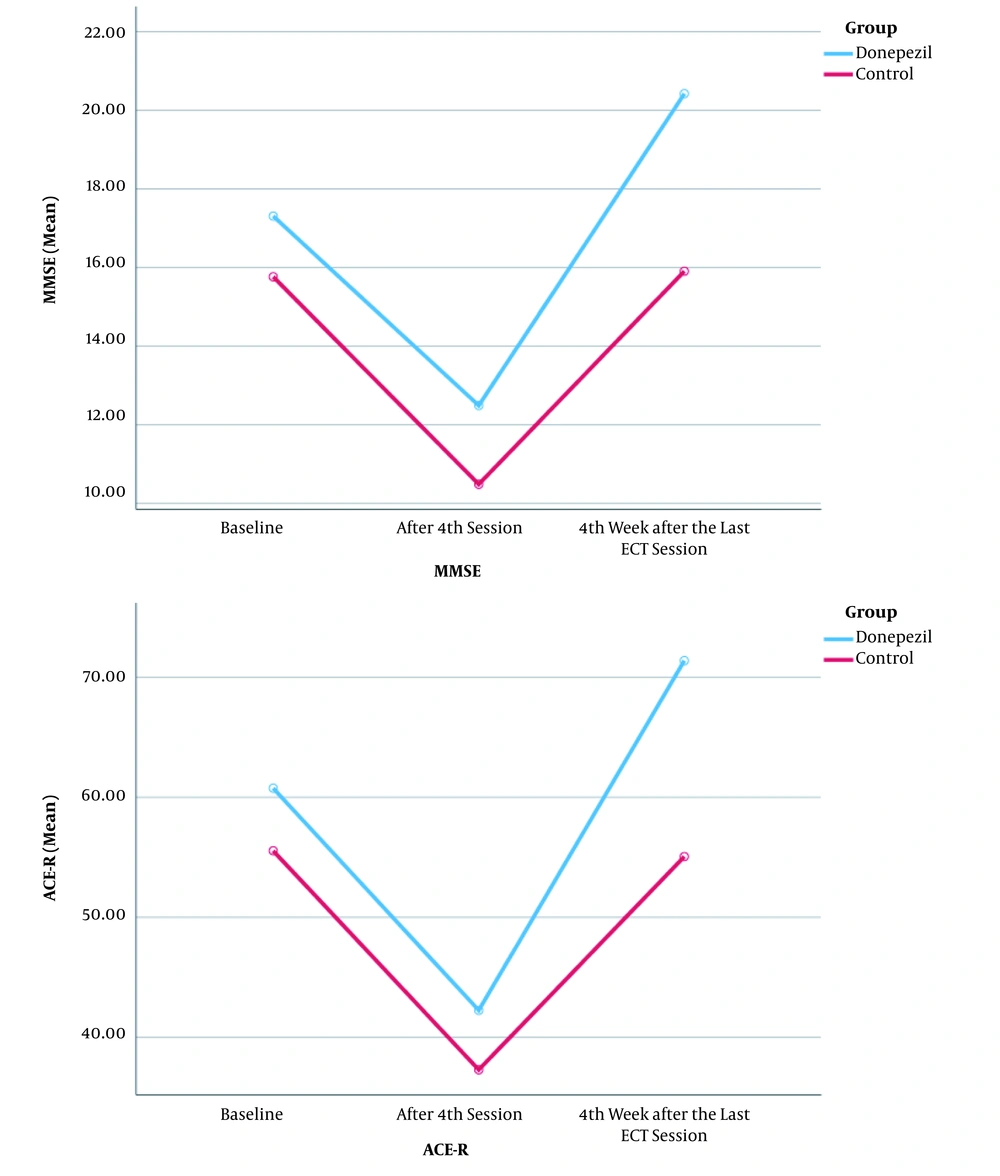
The significance level of the main effect of time was < 0.001 (F = 49.22, df = 1.67), indicating that MMSE improved significantly from the beginning to the fourth week after the last ECT session. The total scores of MMSE improved from 17.10 ± 6.01 to 20.20 ± 5.06 in the donepezil groups from the beginning to four weeks after the last ECT session (time effect).
The mean score (± SD) of MMSE in the donepezil groups was higher than that of the placebo groups (F = 5.13, df = 1, P = 0.026) (treatment effect). However, no difference was observed between the male and female groups (F = 1.59, df = 1, P = 0.210). Furthermore, the gender-time interaction was no significant (F = 2.19, df = 1.67, P = 0.123), suggesting that the male and female groups did not differ significantly at the beginning and four weeks after the last ECT session (Table 2).
| Parameter | Baseline | AFTER 4TH Session | 4th Week After Last ECT Session | P-Value Within Group | P-Value Between Groups (Treatment Effect) | |
|---|---|---|---|---|---|---|
| Time-Treatment Effect | Time Effect | |||||
| MMSE | F = 16.74, df = 1.67, P < 0.001 | F = 49.22, df = 1.67, P < 0.001 | F = 5.13, df = 1, P = 0.026 | |||
| Donepezil | 17.10 ± 6.01 | 12.39 ± 5.98 | 20.20 ± 5.06 | |||
| Control | 15.97 ± 6.10 | 10.58 ± 6.18 | 16.13 ± 5.41 | |||
| ACE-R (Total score) | F = 50.13, df = 1.86, P = < 0.001 | F = 96.76, df = 1.86, P < 0.001 | F = 5.74, df = 1, P = 0.019 | |||
| Donepezil | 60.18 ± 18.07 | 42.00 ± 15.11 | 70.89 ± 13.42 | |||
| Control | 56.13 ± 21.16 | 37.56 ± 19.16 | 55.56 ± 18.57 | |||
| ACE-R (attention subscale) | F = 26.19, df = 2, P < 0.001 | F = 60.37, df = 2, P < 0.001 | F = 2.39, df = 1, P = 0.125 | |||
| Donepezil | 11.50 ± 3.43 | 7.47 ± 3.11 | 13.27 ± 2.29 | |||
| Control | 10.82 ± 4.41 | 7.30 ± 3.94 | 10.76 ± 3.81 | |||
| ACE-R (memory subscale) | F = 35.97, df = 1.70, < 0.001 | F = 50.51, df = 1.70, P < 0.001 | F = 7.57, df = 1, P = 0.007 | |||
| Donepezil | 13.39 ± 5.14 | 8.66 ± 3.25 | 16.72 ± 4.00 | |||
| Control | 12.21 ± 6.41 | 7.36 ± 4.89 | 11.86 ± 5.81 | |||
| ACE-R (verbal fluency subscale) | F = 15.38, df = 1.87, P < 0.001 | F = 37.17, df = 1.87, P < 0.001 | F = 6.55, df = 1, P = 0.012 | |||
| Donepezil | 5.35 ± 2.28 | 3.54 ± 1.87 | 6.75 ± 2.24 | |||
| Control | 4.73 ± 2.77 | 3.00 ± 2.37 | 4.93 ± 2.41 | |||
| ACE-R (language subscale) | F = 17.10, df = 1.77, p = 0.001 | F = 23.86, df = 1.77, P < 0.001 | F = 4.13, df = 1, P = 0.045 | |||
| Donepezil | 18.18 ± 5.55 | 13.45 ± 4.76 | 20.68 ± 3.98 | |||
| Control | 17.10 ± 6.13 | 12.30 ± 6.62 | 16.80 ± 5.57 | |||
| ACE-R (Visuospatial subscale) | F = 7.63, df = 1.62, P = 0.002 | F = 23.04, df = 1.62, P < 0.001 | F = 3.34, df = 1, P = 0.071 | |||
| Donepezil | 11.75 ± 4.29 | 8.85 ± 4.50 | 13.45 ± 3.25 | |||
| Control | 11.23 ± 3.95 | 7.58 ± 4.42 | 11.19 ± 3.76 | |||
4.2.2. Secondary Outcome (ACE-R)
The Greenhous-Geisser correction (p = 0.035 for Mauchly’s test of Sphericity) was used to assess the ACE-R changes over time. First, the treatment-time interaction was evaluated, and the result was significant (F = 50.13, df = 1.86, P = < 0.001), indicating that the ACE-R improvement at the beginning and the fourth week after the last ECT session was significantly different in the donepezil and placebo groups (Table 2). Like MMSE, different effects cannot be considered for time and treatment.
There was no difference in the male and female groups by ACE-R (F = 1.06, df = 1, P = 0.305). Gender had not significantly affected an increase of ACE-R between baseline and last time. In other words, the (gender-time) interaction was not significant (F = 2.40; df = 1.86; P = 0.098).
Regarding the attention subscale, the significance level of Mauchly’s test of Sphericity was P > 0.05 (P = 0.992). Accordingly, Sphericity test was used to report the effects of time and time-treatment. The significance levels of Mauchly’s test of Sphericity were P < 0.05 for the memory (P < 0.001), verbal fluency (P = 0.041), language (P = 0.002), and visuospatial (P < 0.001) subscales. Accordingly, Greenhouse-Geisser correction was used to report the time and time-treatment effects. Table 2 shows the time-treatment, time, and treatment effects of the subscales. The gender-time interaction was significant in none of the ACE-R subgroups. Moreover, gender did not affect the differences in the mean scores (± SD) of the ACE-R subgroups in the donepezil and placebo groups. Figure 2 compares the trends of MMSE and ACE-R changes over time in the donepezil and placebo groups.
5. Discussion
This study documented the efficacy of donepezil over the placebo in improving cognitive status in patients receiving ECT. The mean scores of MMSE and ACE-R revealed significant post-intervention improvement in the donepezil group compared to the placebo group.
To the best of our knowledge, few studies have examined the efficacy of donepezil in improving the ECT-induced cognitive complications. In accordance with Prakash’s et al. study (19) and a case report (18), our findings indicate that donepezil is beneficial in reducing the cognitive side-effects of ECT. Roa et al. (18), in their case report showed that donepezil attenuates cognitive complications in a patient undertaking ECT. In Prakash’s et al. (19) study, 45 patients with different psychiatric diagnoses were evaluated. The participants received donepezil or placebo from two hours before ECT until three days after the last ECT session. In the donepezil group, faster post-ECT cognitive recovery was observed in comparison to the placebo group. In line with our study, a meta-analysis indicated that cognitive enhancers might improve cognitive function and decrease cognitive side-effects induced by ECT (25).
Unlike our findings, Shams-Alizadeh et al. (20) reached different findings. Their study encompassed 78 patients with different diagnoses (e.g., schizophrenia, MDD, BID, and others), and they administered donepezil during the ECT period and evaluated cognitive performance 48 hours after the last ECT session. In contrast, we continued prescribing donepezil for one month after the last ECT session and then assessed the patient’s cognitive functions. It seems that it takes a longer time to observe the cognitive-enhancing effect of donepezil. Dutta et al. reported the insignificant efficacy of donepezil vs. placebo in terms of reducing ECT-induced cognitive deficits in 30 patients with schizophrenia or MDD (21). The small number of the participants or the inclusion of the patients with different disorders (namely MDD and schizophrenia) might explain the inconsistency of the findings.
Our findings support the possible involvement of the cholinergic system in cognitive side-effects induced by ECT. Adjunct donepezil decreased the frequency of such side-effects (26). In the donepezil group, all patients completed the study. Consistent with previous studies, the patients receiving donepezil reported no remarkable complication (19, 20).
In our RCT, we prescribed a dose of 5 mg/d donepezil; hence, higher doses are recommended to be considered in future studies. In this study, although the baseline cognitive status was not significantly different between the two groups, we could not match the participants regarding the number of episodes and the disease duration. Another limitation of this study was the non-uniformity of the drugs between the two groups during ECT. However, we spared our efforts to minimize this possible effects by prescribing selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors in the MDD group and atypical antipsychotics in the bipolar patients. Moreover, randomization would contribute to matching the effects of different drugs, the number of episodes, and the disease duration between the study groups.
5.1. Conclusions
Donepezil co-administrated with ECT may improve the ECT-induced cognitive disturbances.