1. Background
Attention deficit/hyperactivity disorder (ADHD), with a prevalence of 3 - 7%, is one of the most important psychiatric disorders in children (1). The distinguished features of this disorder include such symptoms as inattention, hyperactivity, and impulsivity that do not match with the age of child development (2). There are different approaches to manage ADHD, such as psychotherapy and pharmacotherapy, and the latter one is the standard approach for the reduction of different symptoms of ADHD. Among psychiatric drugs, methylphenidate (MPH) is the most common and safest medication for treating ADHD, so that different studies have used it for treating the related symptoms (3, 4). MPH reduces cognitive deficits through a dopaminergic effect (5). However, about 30% of affected children and adolescents do not respond to MPH (6). So, in recent years, alternative treatments have been considered. Herbal remedies have been considered throughout the history. It is noteworthy that herbal medicines have been widely used worldwide (7). Accordingly, around 80% of people in different parts of the world accept these remedies as effective drugs (8). In the treatment of depression, herbal medicines with important properties such as low risk and milder side effects are considered as alternative treatment options (9-12). In this regard, one of the herbal medicines is Crocus sativus (saffron), which has extraordinary properties such as anti-cancer and anti-platelet effects, antioxidant benefits, and anti-inflammatory medical usage (9). The underlying hypothesis of the effect of saffron on ADHD symptoms is based on psychiatric and clinical evidence that reinforces the hypothesis of dopamine. In other words, saffron increases dopamine by increasing activity and brain waves and plays the role of stimulants (13). Besides, it has antispasmodic, antiseptic, antidepressant, and anticonvulsant therapeutic applications (14). Accordingly, it is believed that this expensive spice can inhibit the reuptake of dopamine and norepinephrine. Noticeably, dopamine and norepinephrine are N-methyl D-aspartic acid (NMDA) receptor antagonists and also GABAA agonists (15, 16).
Scientifically, different studies have demonstrated efficacy of this expensive spice by exact clinical trials. These studies showed that saffron was capable of enhancing memory function and reducing depression symptoms and anxiety (17, 18). Others evaluated the efficacy of MPH and saffron in treating children with ADHD. Their results showed no significant difference between the two groups under treatment with MPH and saffron (19). Some research also evaluated the effectiveness of other herbal medicine such as Valerian root, the results of which were significant (20).
2. Objectives
Since individuals with ADHD have increased rates of other psychiatric disorders such as alcohol-related disorders, drug addiction, divorce, family conflicts, and deadly accidents in adulthood (21), we hypothesized that combining saffron with MPH would have higher efficacy than MPH alone. It was assumed that this herbal supplement would be able to improve the effectiveness of treatment. Given these studies and the lack of comparative research in this field, the authors of this article conducted a comparative study to evaluate MPH and its combined use with saffron among children and adolescents suffering from ADHD.
3. Methods
3.1. Procedure and Study Setting
The present clinical trial was a randomized, double-blind, parallel-group study conducted over an 8-week period (from February 2020 to April 2020) in Mehr Psychiatric Hospital affiliated to Lorestan University of Medical Sciences (LUMS), Iran. The ethics committee of LUMS approved all study protocols (IR.LUMS.REC.1398.227), and the study was registered in Iranian Registry of Clinical Trials (IRCT20190602043790N2). The study was conducted according to the principles of the Declaration of Helsinki. Patients' symptoms were measured at the baseline time and weeks four and eight. To meet the ethical codes, all the steps and study objectives were described to the children. Adolescents and their parents. Written informed consent was obtained from the children and adolescents or their parents. Moreover, all necessary explanations concerning the right to withdraw from the study for any reason were provided to the participants and their parents.
3.2. Sample Size
Through calculating the mean difference (MD = 3) and standard deviation (SD = 3) in the pilot study of ADHD-RS-IV (Attention-Deficit/Hyperactivity Disorder Rating Scale-IV) and regarding the statistical power of 80% (P < 0.05), 32 patients were needed for each group (n = 64). Considering 10% attrition rate, the statistics specialist proposed 35 patients to be included in each group (n = 70).
3.3. Participants
Recruitment process was performed among patients of both sexes aged 6 - 16 years admitted to the outpatient wards. All patients met the necessary criteria for ADHD diagnosis according to DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition). All the eligible patients had total and/or subscale scores on ADHD-RS-IV and at least SD = 1.5 from the norms (23). Before any intervention, the child psychiatrist diagnosed ADHD based on the DSM-5 criteria for all participants and took a complete medical history. A simple randomization method was used, and the unit of randomization was individual. Using the table of random numbers, patients were randomly divided into two groups: MPH or a combination of MPH and saffron. Even numbers were considered for the MPH group and odd numbers for the MPH and saffron group. Random allocation was done by the researcher; the clinical caregiver and data analyzer were not aware of the allocation of patients.
Exclusion criteria included psychiatric comorbidities (except for oppositional defiant disorder), seizure, cardiac disease, using any external matter that might interfere with saffron, history of allergy to saffron, and a systolic blood pressure (SBP) over 125 mm Hg. The physiological examinations included body mass index (BMI), pulse rate, and blood pressure.
3.4. Interventions
The samples were randomly assigned into two parallel groups. The first group only received MPH at the dose of 0.3 - 1 mg/d. According to the protocol, MPH was prescribed during the trial at the below doses: 10 mg/d (5 mg for morning and midday equally) in week 1; 20 mg/d (10 mg for morning and midday equally) in week 2; and 20 mg/d (for < 30 kg and 30 mg/d for > 30 kg; 10 mg for morning, midday, and evening equally) in week 3 and thereafter. The MPH plus saffron group received previous prescription with saffron capsules at a dose of 20 - 30 mg/d according to the BMI (20 and 30 mg/d for < 30 and > 30 kg, respectively).
3.5. Instrument
Parent and teacher ratings of ADHD-RS-IV was used for evaluating behavioral changes in patients. This tool provided valid measurement of attention and behavioral problems in children and adolescents with ADHD over the past six months and took about five minutes to be completed (22, 23). This instrument is consistent with the DSM-V criteria for diagnosis of ADHD (24). In this tool, 18 distinctive symptoms of ADHD are assessed by a 4-point Likert scale. Psychometric characteristics of this measure were confirmed (23). In this questionnaire, parents and teachers are asked to choose one of the options (frequency of behavior). The first nine items are related to inattention symptoms, and the second nine items are related to hyperactivity and impulsivity symptoms. Cronbach's alpha was calculated at 0.90 for the school and home versions (24). Furthermore, construct validity of the combined subscale for the two groups of respondents was reported as 60 and 65% for parents and teachers, respectively (24).
3.6. Safety
All participants were free to withdraw from the trial at any stage. Also, sufficient information was provided to inform the members of the research team in case of any unexpected side effects. Throughout the trial, side effects were exactly investigated using a checklist; 25 side effects, including neurological and psychological ones, as well as other types were recorded (25). Over the course of eight weeks, there was no dropout due to different side effects.
3.7. Data Analysis
For data analysis, the IBM SPSS Statistics (version 21) (IBM Corporation, Armonk, NY) was used. Number of patients and percentages were considered as categorical variables and mean, and SD were counted as continuous variables. To compare the trend of changes in ADHD Rating Scale scores during eight weeks of treatment, the general linear model (GLM) repeated measures was used. Between-subject and within-subject factors were counted for study groups and times of measurements, respectively. Greenhouse–Geisser correction was performed for reporting the degrees of freedom (DF) if Mauchly’s test of sphericity was statistically significant. Moreover, for evaluating effectiveness of each protocol in reducing ADHD symptoms, one-way analysis of variance (ANOVA) was performed. T-test was also used to compare changing the status of scores from baseline in both groups. Two statistical methods, including Fisher’s exact test and chi-square test, were also used for comparing the categorical variables. Also, in all stages of analysis, a P-value < 0.05 was considered as the significant level.
4. Results
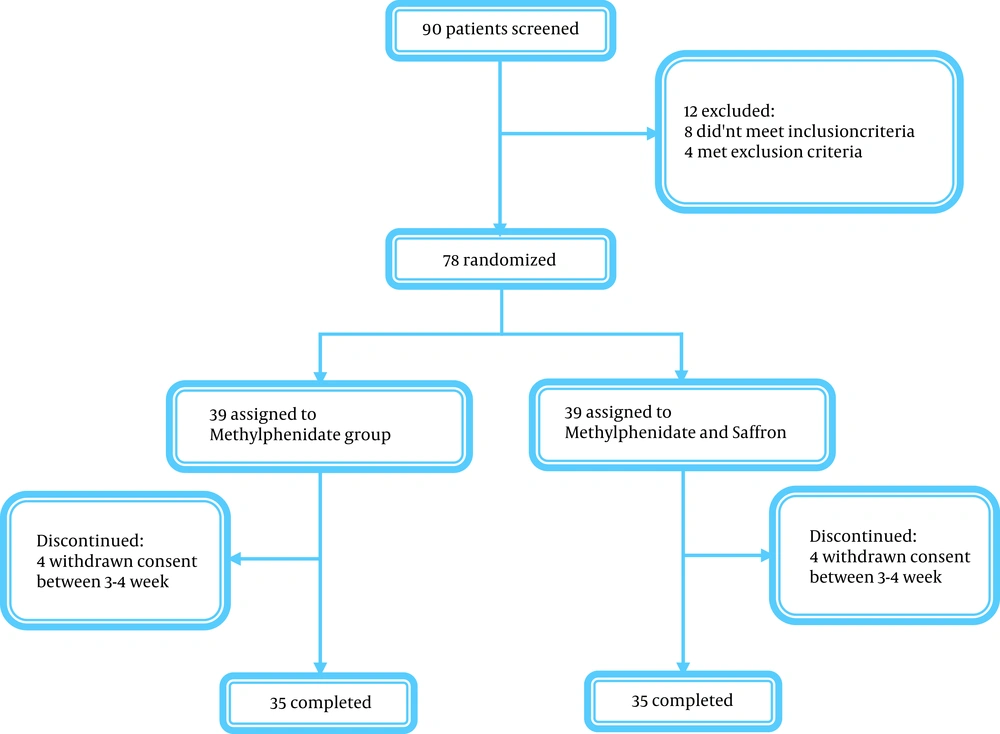
In this study, out of a total of 90 children and adolescents aged 6 - 16 years, 12 patients were excluded due to not meeting the inclusion criteria. In this way, 39 patients were randomly assigned to MPH group and 39 to the MPH plus saffron group. In weeks 3 - 4, eight subjects refused to participate in the trial. Thus, in every group, 35 patients finished the trial (Figure 1). Also, there was no significant difference between the two groups in terms of baseline characteristics such as age (Table 1).
| Variables | N | Mean | SD | P-Value |
|---|---|---|---|---|
| Age | 0.435 | |||
| MPH | 35 | 11.03 | 2.31 | |
| MPH & Saffron | 35 | 10.57 | 2.56 | |
| Parent inattention baseline | 0.261 | |||
| MPH | 35 | 19.13 | 2.98 | |
| MPH & Saffron | 35 | 19.99 | 3.33 | |
| Parent hyperactivity baseline | 0.824 | |||
| MPH | 35 | 20.11 | 3.16 | |
| MPH & Saffron | 35 | 19.93 | 3.47 | |
| Teacher inattention baseline | 0.793 | |||
| MPH | 35 | 18.51 | 3.00 | |
| MPH & Saffron | 35 | 18.71 | 3.20 | |
| Teacher hyperactivity baseline | 0.602 | |||
| MPH | 35 | 18.39 | 2.65 | |
| MPH & Saffron | 35 | 18.77 | 3.38 | |
| Parent total baseline | 0.618 | |||
| MPH | 35 | 39.27 | 5.23 | |
| MPH & Saffron | 35 | 39.95 | 6.07 | |
| Teacher total baseline | 0.661 | |||
| MPH | 35 | 36.94 | 4.82 | |
| MPH & Saffron | 35 | 37.52 | 6.07 |
4.1. Parent ADHD-RS-IV
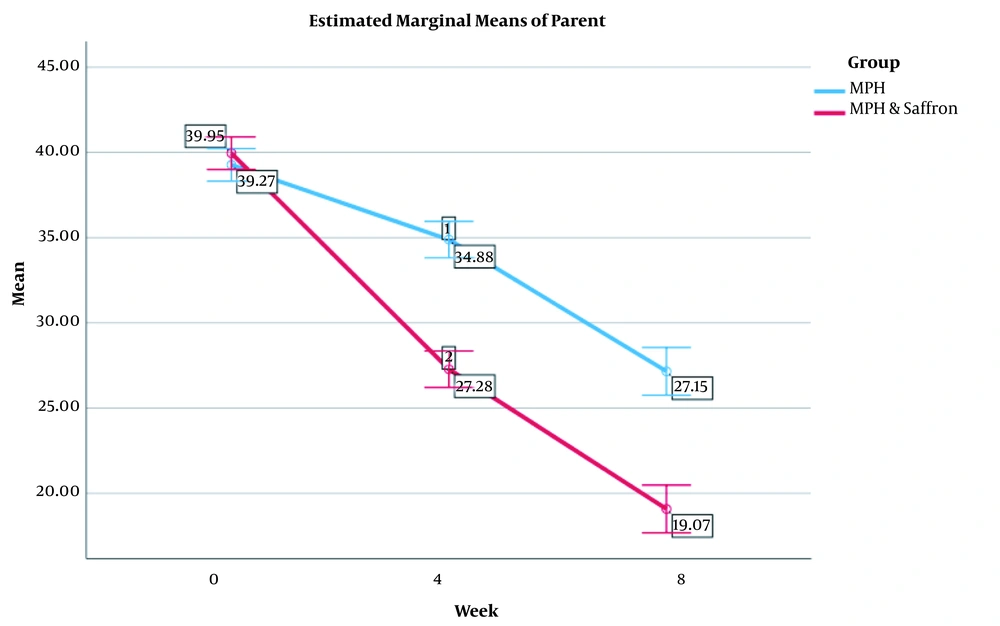
As the results indicated, in baseline stage, no significant difference was observed between the two study groups in parent ADHD Rating Scale scores (39.27 ± 5.23 for MPH and 36.94 ± 4.82 for MPH plus saffron group, df = 68, P < 0.05). The groups also proved to be different according to the results of GLM. Moreover, the effect of interaction between time and treatment was similar across time (Figure 2). In each group, the trend of the effect of time × treatment interaction was similar. Regarding the subscales of hyperactivity and inattention, Greenhouse-Geisser corrected GLM repeated measures indicated no change in between-subject groups. This report was similar for the two subscales. Therefore, the trend analysis in the fourth and eighth weeks showed significant differences in the scores of parent ADHD Rating Scale among the two groups (P < 0.05). Also, post hoc comparisons showed a significant reduction in Parent ADHD scores in the fourth and eighth weeks in both groups (P < 0.05). After four weeks of treatment, the patients achieved the desired result. The average score of parents in the MPH with saffron group was lower than the average total score of the parents in the MPH group after eight weeks (P < 0.05) (Table 1).
4.2. Teacher ADHD-RS-IV
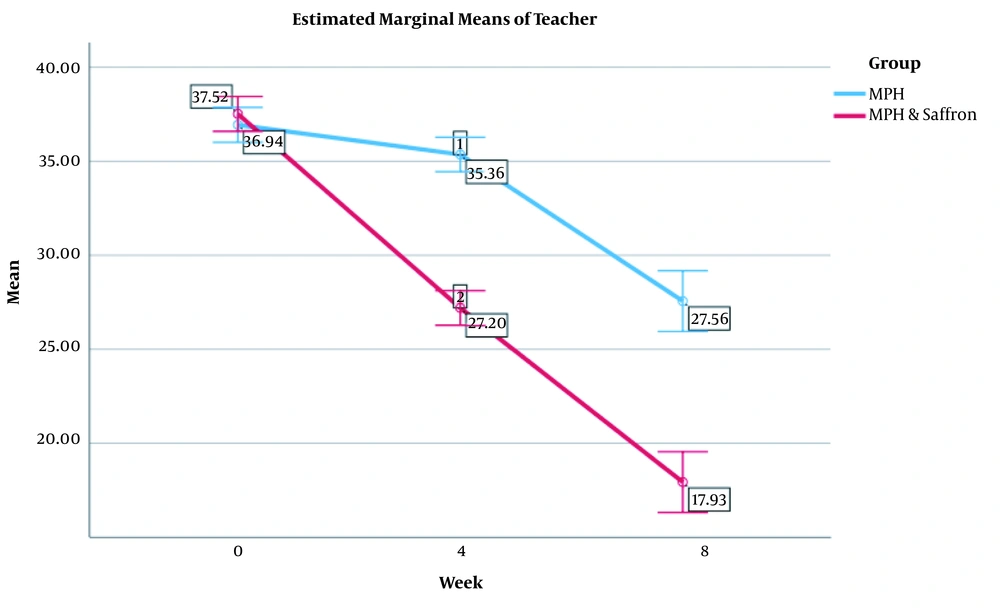
As the results indicated, in baseline stage, no significant difference was observed between the two study groups in teacher ADHD Rating Scale scores (36.94 ± 4.82 and 37.52 ± 6.07 for MPH and MPH plus saffron group, respectively) (df = 68, P < 0.05). According to the results of GLM repeated measures, there was no change in between-subject groups. Moreover, effect of time × treatment interaction was similar across time (Figure 3). In each group, the trend of the effect of time × treatment interaction was similar for two subscales, including hyperactivity and inattention. Accordingly, MPH improved ADHD symptoms, but MPH plus saffron had excellent effect on the reduction of symptoms. This report was similar for two subscales. There was a significant difference between the two groups in the scores of teacher ADHD Rating Scale at the fourth and eighth weeks. In other words, the difference between the two groups after four weeks was obtained in the mean scores of hyperactivity, inattention, and total score. Post hoc comparisons showed significant reductions in Teacher ADHD scores at weeks 4 and 8 between the two groups (P < 0.05). Accordingly, the difference between baseline characteristics in weeks 4 and 8 was significant among the two groups (P < 0.05). After four weeks of treatment, patients achieved the desired result (Table 2).
| Variables | MPH | MPH & Saffron | P-Value |
|---|---|---|---|
| Parent inattention week 4 | 17.69 ± 3.24 | 13.54 ± 3.46 | 0.000 |
| Parent inattention week 8 | 13.77 ± 4.7 | 9.21 ± 3.71 | 0.000 |
| Parent hyperactivity week 4 | 17.18 ± 3.92 | 13.72 ± 3.43 | 0.000 |
| Parent hyperactivity week 8 | 13.37 ± 5.16 | 9.83 ± 3.51 | 0.001 |
| Parent total week 4 | 34.88 ± 6.6 | 27.28 ± 6.06 | 0.000 |
| Parent total week 8 | 27.15 ± 9.46 | 19.07 ± 6.9 | 0.000 |
| Teacher inattention week 4 | 17.91 ± 2.92 | 13.4 ± 2.72 | 0.000 |
| Teacher inattention week 8 | 13.49 ± 5.71 | 9.19 ± 4.03 | 0.001 |
| Teacher hyperactivity week 4 | 17.41 ± 3.73 | 13.75 ± 2.38 | 0.000 |
| Teacher hyperactivity week 8 | 14.03 ± 5.76 | 8.69 ± 3.73 | 0.000 |
| Teacher total week 4 | 35.36 ± 6.2 | 27.2 ± 4.49 | 0.000 |
| Teacher total week 8 | 27.56 ± 11.26 | 17.93 ± 7.46 | 0.000 |
a Values are expressed as mean ± SD.
4.3. Side Effects
No serious side effects were reported during the study, and all observed side effects were mild to moderate. Furthermore, there was no significant difference between patients in MPH and MPH plus saffron groups in terms of repetition of side effects.
5. Discussion
The results of this study supported the efficacy of the combination of MPH and C. sativus (saffron) over MPH alone in the treatment of patients suffering from ADHD. The results of GLM also showed that combined MPH with C. sativus (saffron) was more effective than MPH alone after 4 - 8 weeks of treatment. Moreover, after four weeks, the effectiveness of treatment was obvious. So, the novelty of this trial was that by prescribing MPH with saffron, the duration of treatment was reduced to four weeks. This reduction in time can be effective in accelerating the treatment process and reducing side effects.
ADHD is widely recognized as a common childhood neurodevelopmental psychiatric disorder, and pharmacological approach is the most accepted approach for its treatment. The Food and Drug Administration (FDA) approved some stimulant drugs like Ritalin, methylin ER, cotempla XR, and metadate CD to treat ADHD; these first-line drugs are the most prestigious remedies. However, for psychiatrists, the foremost drugs are ritalin and dexmethylphenidate, respectively (26). Brain imaging studies have shown that dopamine and norepinephrine pathways are involved in the pathology of ADHD. Dopamine and norepinephrine pathways are also involved in other comorbid disorders such as depression, anxiety, and panic (27-33). Therefore, it is not surprising that by regulating these pathways some improvements occur in mood and concentration of ADHD patients; they also show why MPH is effective for the treatment of ADHD (34). Taken together, the first-line treatment for ADHD fails in up to 20 - 30% of patients suffering from ADHD (35).
This randomized study showed that saffron, as an herbal medicine, could be an effective complementary treatment for ADHD like other disorders such as cancer and metabolic syndromes (36). Saffron possesses antidepressant and anti-inflammatory properties and also has a radical scavenging capability (10). Moreover, some studies have been indicative of its anti-Alzheimer, anti-schizophrenia, and anti-Parkinson properties (37-41).
Considering herbal approach to the treatment of ADHD, studies have shown that saffron is an antidepressant supplement, and it has many marvelous effects in relieving ADHD obvious symptoms. Saffron reduces the symptoms of abnormalities and acts as a safe drug by regulating neurotransmitters and modulating their secretion (10, 22, 42-44). Moreover, saffron has also been shown to exert antidepressant effects in clinical trials. Thus, we hypothesized that saffron could be beneficial in combination with MPH and alleviate symptoms effectively. Similarly, the monoaminergic and glutamatergic systems of saffron (45) supported by rich literature make this plant a complementary medicine capable of treating ADHD.
From a biological standpoint, one of the explanations for the high effectiveness of saffron in combination with MPH is its effect on monoaminergic and glutamatergic systems. Chemical compounds of saffron can improve mood and modulate brain function through monoaminergic and glutamatergic systems (45-48). Furthermore, due to the pathophysiology of ADHD and the involvement of various mechanisms, such as reduced and fluctuated brain volume in some brain areas (prefrontal cortex, cerebellum, and basal ganglia), it seems that these regions are directly involved in ADHD emergence (49). Some researchers believe that saffron can increase the power of neuronal plasticity and neurogenesis or growth of neural tissue of specific brain regions, such as hippocampus, nucleus accumbens, and prefrontal cortex. In fact, neuronal plasticity by increasing brain activity and regulating waves can be effective in keeping the brain of people with ADHD self-regulated. This ability of the brain is also effective in regulating the emotions of sufferers (9). It is noteworthy that combined treatment showed its effectiveness in a shorter period of time compared to MPH treatment alone. So, saffron has a golden effect on shortening the duration of treatment to four weeks, and it has fewer side effects for children and adolescents due to its herbal properties.
This clinical trial had some useful results. A randomized method and a double-blind design were among its advantages. Nonetheless, there were some limitations, that is, lack of a placebo-controlled trial and lack of a follow-up period. These limitations should be addressed in future trials. As a result, future randomized placebo-controlled trials are recommended to evaluate patients during the process of treatment more rigorously.
5.1. Conclusions
In this study, although MPH reduced ADHD symptoms, the combination of MPH and saffron proved to be more effective than MPH alone. In addition, by prescribing combined treatment, the duration of treatment can be reduced to four weeks. Considering the positive effect of saffron, future studies should consider its use for a broader spectrum of psychiatric disorders like anxiety disorders, depression, etc.