1. Background
Obsessive-compulsive disorder (OCD) is characterized by repetitive thoughts or behaviors obliging a person to perform unusual reactions and release unpleasant, irritating, and anxious feelings (1). This bothersome condition is associated with considerable morbidity and has a prevalence of 0.3% to 3.1% worldwide (2).
The serotoninergic system dysfunction is the principal pathway playing a role in the pathophysiology of OCD. Diverse genes are associated with serotonin neurotransmission, metabolism, and reuptake in the central nervous system, and their role has been investigated in OCD pathophysiology. Selective serotonin reuptake inhibitors (SSRIs) are included in the primary medical regimens considered for OCD management (3, 4).
Glutamate, a ubiquitous excitatory neurotransmitter, has been noted to have a role in the pathophysiology of OCD, particularly through the cortico-striatothalamo-cortical circuitry (5). Neuroimaging examinations and cerebrospinal fluid (CSF) and genetic studies confirmed that glutaminergic dysfunction was associated with OCD presentations, proposing that patients might benefit from the remedies modulating glutamatergic neurotransmission (6).
The most recent theory about the etiology of OCD refers to a proinflammatory cytokine storm in the central nervous system. This hypothesis remarks that patients with OCD basically have a low-grade inflammation, which can be exacerbated early in life. Thereafter, through certain pathways such as infections or psychological stress, proinflammatory mediators bind to the cerebral vascular endothelium and in turn cause the overexpression and release of secondary biomarkers such as prostaglandins and nitric oxide, leading to local tissue damage (5). This opinion has been reinforced through neuroimaging and genetic studies (7, 8).
Selective serotonin reuptake inhibitors include the first pharmacological choice for OCD treatment. Although the introduction of SSRIs opened an encouraging window for treating this chronic disorder, 40% - 60% of OCD patients do not adequately respond to these drugs. Moreover, 30% - 40% of responders may resent continuing treatment to resolve the residuals of their symptoms (5, 6). The fact about the inadequate response of OCD patients to pharmacological agents clarifies the necessity of further studies, including the use of augmentation therapy and switching to novel agents (1, 9).
Despite accessibility to various treatment approaches for OCD, complete remission is notably rare (10). A variety of medical therapies, in addition to psychotherapy and cognitive behavior therapy (CBT), as the most effective one, have been administered. Nevertheless, well-responsiveness to these approaches was inadequate, and numerous OCD patients experience refractory phases (11).
Clonidine is an alpha-2 adrenergic agonist showing beneficial effects for OCD treatment. However, studies in this regard are limited, and this notion has not been assessed in controlled trials (12, 13). Hollander et al. (14) presented a significant role for clonidine in improving OCD symptoms and proposed a theory about the traces of neuroendocrine disorders in OCD pathophysiology. Khanna et al. (15) also described promising outcomes for clonidine in OCD management, while Hewlett et al. (16) found that clonidine was not adequately effective.
2. Objectives
The current study aims to assess the efficacy and side-effects of clonidine augmentation therapy in the treatment of OCD in a controlled trial for the first time. The primary outcome of this study was to evaluate patients’ responses to the medication using validated scales designed to assess the severity of OCD symptoms. Furthermore, patients were evaluated in terms of drug-related adverse effects as the secondary outcome.
3. Methods
3.1. Study Population
The current study was a double-blinded randomized clinical trial conducted on 57 OCD patients referred to the outpatient OCD clinics of Nour and Ali-Asghar hospitals affiliated with Isfahan University of Medical Sciences from May 2016 to March 2019.
Inclusion criteria were as follows:
(1) Documented diagnosis of OCD based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (17); (2) at least 12 weeks of treatment with the maximal dose of SSRIs or clomipramine; and (3) moderate to severe symptoms of OCD (i.e., a score above 16 based on the Yale-Brown Obsessive-Compulsive scale (Y-BOCS)) (18, 19).
The diagnosis of primary psychotic or mood disorders, drug abuse or addiction, chronic uncontrolled medical conditions (e.g., diabetes mellitus, hypertension, cardiovascular diseases, etc.), a previous history of clonidine use, pregnancy or lactation, a history of epilepsy/seizure or suicidal thoughts, and the use of beta-blockers or any other treatment for concurrent psychological disorders were considered as exclusion criteria.
Pregnancy at any stage during the study, requirement for using additional pharmaceutical or non-pharmaceutical medications during the study, and unwillingness to adhere to the study protocol at any stage of the study were other exclusion criteria.
The Ethics Committee of Isfahan University of Medical Sciences approved the study protocol. Besides, this study was registered at the Iranian Registry of Clinical Trials and approved under the code of IRCT20110918007582N2. After that, the study process was explained to all the participants, and they were reassured about the confidentiality of their personal information. All the participants were requested to sign a written consent form before participating in the study.
The sample size was calculated according to the below formula in which σ-1, σ-2, α, β, and d were regarded as 0.52, 0.33, 0.05, 0.2, and 0.35, respectively. Accordingly, the estimated sample size for each group was obtained 25, but due to assumed withdrawals, 30 patients were recruited for each group.
The diagnosis of OCD was made according to DSM-V by an expert psychiatrist attending at Isfahan University of Medical Sciences. Study participants were selected through block randomization sampling until achieving the desired number of participants. For this, the patients who met the inclusion criteria were entered into a block consisting of four patients in a specified order. The total number of blocks was 15. The members of each block were assigned with a particular number using randomization crosstabs. Two patients were allocated to the control group, and remaining patients were assigned to the intervention group.
3.2. Intervention
The intervention group was treated with clonidine (Toliddaru, Iran). The treatment was initiated with a daily dose of 0.1 mg that increased 0.2 mg per week based on patients’ tolerance until reaching the dose of 1 mg/day. Due to the paucity of knowledge regarding the appropriate dose of clonidine for treating OCD, both the initial and maximal administered doses were determined according to a study by Hewlett et al. (16).
The control group underwent treatment with placebo with similar shape, size, and color to clonidine, made by Raha Company, Iran. The order of placebo administration was similar to the intervention group in order to minimize possible bias.
Patients’ primary treatments, including SSRIs or clomipramine, continued with the same dose used before participation in this study. Augmentation treatments were initiated as soon as patients met the criteria for refractory OCD diagnosis. The patients were treated for 12 weeks.
The participants’ complete adherence to the medication was checked by a psychiatry resident who was responsible for making weekly calls. The participants were blinded to the type of medication that they were using during the study. Furthermore, the psychiatrist who interviewed the participants was blinded to the type of treatment used by the interviewee.
3.3. Tools of Assessment
3.3.1. The Yale-Brown Obsessive Compulsive Scale
This scale was initially proposed by Goodman et al. Thereafter, this 10-item scale, which is used to assess OCD severity, was translated to Persian and validated with the internal consistency values of 0.97 and 0.95 for the symptom checklist and symptom severity scale, respectively. The test-retest reliability of the Persian version was reported 0.99 (18).
3.3.2. The Clinical Global Impression
This is an old popular instrument to assess psychiatric disorders globally. This questionnaire assesses both the severity and improvement of various psychiatric disorders. The reliability of this instrument was 0.91, and its score ranges from one to seven, indicating normal, borderline, mild, moderate, marked, severe, and highly severe mental illness, respectively (20, 21).
3.4. Outcomes
The primary outcome of the study was response to the medication, which was assessed using the validated tools mentioned above. All participants were interviewed using the YBOCS scale at the baseline and four, eight, and 12 weeks after the intervention. Furthermore, the Clinical Global Impression-Severity scale (CGI-S) was completed for all patients at the baseline and within eight and 12 weeks following the treatment. Besides, the incidence of any adverse effect related to the use of clonidine was recorded in a checklist.
3.5. Statistical Analysis
The data obtained were entered into SPSS-20 (IBM, Chicago, the United States) for statistical analysis. The Kolmogorov-Smirnov test was applied to assess the normality of data distribution. The data were described as mean and percentages. Bonferroni and repeated-measure ANOVA were utilized for data analysis. A P-value of less than 0.05 was considered as the significance level.
4. Results
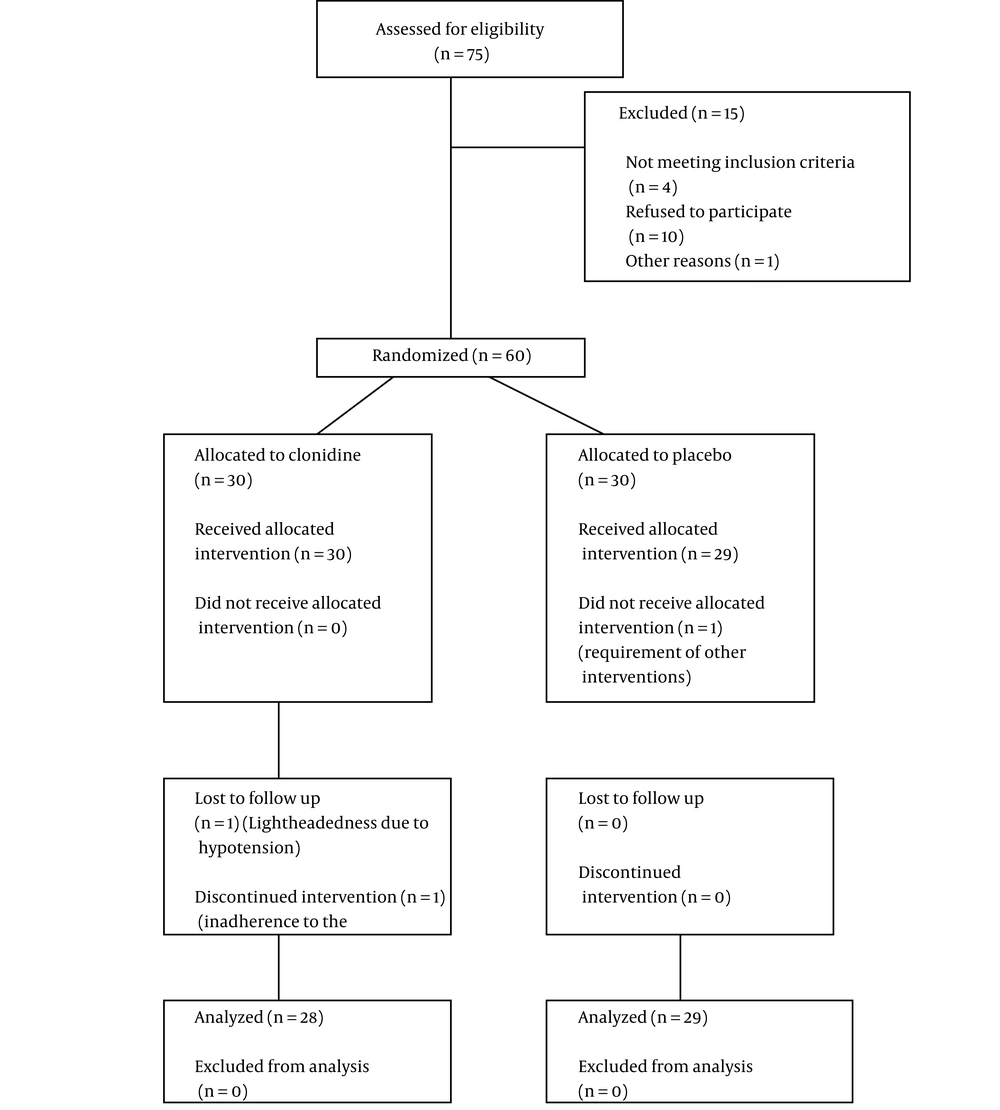
The current clinical trial was conducted on 60 OCD patients. At the onset of the study, each of the intervention and placebo groups contained 30 members. One of the members of the placebo group left the study because of a requirement for electroconvulsive therapy due to the exacerbation of his symptoms and suicidal thoughts. In the intervention group, two participants left, one because of clonidine-associated adverse effects (hypotension requiring medical attention due to significant lightheadedness), and the second due to non-adherence to the study protocol (Figure 1).
The studied population consisted of 37 females (64.91%) with mean age of 36.34 ± 9.85 years. The two studied groups were similar in terms of age (P-value = 0.83) and gender distribution (P-value = 0.64). Detailed information has been demonstrated in Table 1.
The study population was assessed using the CGI and YBOC scales at four-week intervals. According to the Bonferroni test, a remarkable change was seen in the CGI score in both the clonidine-treated and placebo groups (P-value < 0.001); however, there was no significant difference between the two groups (P-value = 0.22). Regarding the YBOC score, a significant change was noticed in both the intervention and control groups after 12 weeks of the treatment, according to the Bonferroni test (P-value < 0.001). A comparison between the two groups showed no significant difference (P-value = 0.06). Detailed information has been presented in Table 2.
| Time Points | Clonidine Group, Mean ± SD | Placebo Group, Mean ± SD | P-Value |
|---|---|---|---|
| Clinical Global impression | |||
| Baseline | 3.89 ± 1.57 | 4.10 ± 1.61 | 0.62 |
| 4th Week | 3.57 ± 1.47 | 3.72 ± 1.43 | 0.69 |
| 8th week | 2.71 ± 1.21 | 3.38 ± 1.42 | 0.06 |
| 12th week | 2.29 ± 1.18 | 3.07 ± 1.51 | 0.03 |
| P-value | < 0.001 | < 0.001 | |
| P-value | 0.22 | ||
| Yale-Brown Obsessive Compulsive scale | |||
| Baseline | 27.61 ± 8.08 | 28.69 ± 7.44 | 0.60 |
| 4th Week | 24 ± 7.19 | 27.59 ± 7.16 | 0.06 |
| 8th week | 22.18 ± 6.95 | 26.72 ± 7.10 | 0.02 |
| 12th week | 20.25 ± 6.80 | 25.45 ± 7.35 | 0.008 |
| P-value | < 0.001 | < 0.001 | |
| P-value | 0.06 | ||
Comparison of Clinical Global Impression and Yale-Brown Obsessive Compulsive Scale Scores Between the Intervention and Placebo Groups
A comparison between the intervention and placebo groups regarding treatment-associated complications showed that drowsiness, hypotension, dry mouth, and constipation were significantly higher among the patients treated with clonidine than the control subjects after four weeks of the treatment (Table 2, P-value < 0.05). At eight-week post-intervention, remarkably higher rates of hypotension and dry mouth were observed in the clonidine-treated group as compared to the placebo group (P-value < 0.05). However, other adverse effects, including drowsiness, dizziness, and nausea, were not significantly different between the two groups. Also, drug-related complications were not significantly different between the two groups. Table 3 presents further data in detail.
| Time Points | No. (%) | P-Value | |
|---|---|---|---|
| Clonidine | Placebo | ||
| Drowsiness | |||
| 4th week | 16 (57.1) | 7 (24.1) | 0.011 |
| 8th week | 9 (32.1) | 4 (13.8) | 0.099 |
| 12th week | 0 | 0 | - |
| Hypotension | |||
| 4th week | 13 (46.4) | 6 (20.7) | 0.039 |
| 8th week | 5 (17.9) | 0 | 0.017 |
| 12th week | 1 (3.6) | 0 | 0.305 |
| Dry mouth | |||
| 4th week | 9 (32.1) | 3 (10.3) | 0.044 |
| 8th week | 8 (28.6) | 1 (3.4) | 0.009 |
| 12th week | 1 (3.6) | 1 (3.4) | 0.980 |
| Dizziness | |||
| 4th week | 8 (28.6) | 3 (10.3) | 0.081 |
| 8th week | 6 (21.4) | 2 (6.9) | 0.114 |
| 12th week | 2 (7.1) | 2 (6.9) | 0.971 |
| Nausea | |||
| 4th week | 6 (21.4) | 4 (13.8) | 0.449 |
| 8th week | 0 | 0 | - |
| 12th week | 0 | 0 | - |
| Constipation | |||
| 4th week | 6 (21.4) | 1 (3.4) | 0.039 |
| 8th week | 1 (3.6) | 0 | 0.305 |
| 12th week | 0 | 0 | - |
Comparison of Drug-related Complications Between the Intervention and Placebo Groups
Table 4 shows the global improvement of the patients. There was no statistically significant difference between the two groups regarding satisfaction with the treatment (P-value = 0.10).
| Clonidine, No. (%) | Placebo, No. (%) | |
|---|---|---|
| 1 | 7 (25) | 3 (10.3) |
| 2 | 12 (42.9) | 6 (20.7) |
| 3 | 6 (21.4) | 13 (44.8) |
| 4 | 2 (7.1) | 5 (17.2) |
| 5 | 1 (3.6) | 2 (6.9) |
Global Improvement of the Patients Following Treatment with Placebo Versus Clonidine (P-Value = 0.10)
5. Discussion
The incidence of OCD has dramatically increased during recent years, causing a serious global concern. Inadequate therapeutic responses to medical and even non-medical treatments have delivered OCD management a significant challenge for psychiatrists (22-24).
A literature review shows that varieties of neurotransmitters are responsible for OCD development and progression; therefore, combinational therapeutic approaches based on the agents acting on different neural receptors are rational. Augmentation therapy is defined as adding a new agent with different pharmacological effects to routine therapeutic regimens (25, 26). Augmentation therapy has been suggested to lead to better responses to medical treatments in OCD (24).
The therapeutic efficacy of clonidine, as an alpha-adrenergic blocker, has not been yet evaluated in OCD patients in controlled trials. Inadequate information is available on the mechanisms through which clonidine modulates OCD, and there is limited knowledge about the role of noradrenergic system dysfunction in OCD development. Case reports have shown that oral clonidine administration leads to agonistic effects on sympathetic brain nuclei, decreasing cerebral norepinephrine level and patients’ sympathetic activity and controlling their emotional arousal (27).
In the current study, we assessed the effect of clonidine augmentation therapy that was incorporated with the maximal doses of SSRIs/clomipramine for treating OCD patients. Although clonidine-treated patients presented superior outcomes regarding the CGI and YBOC scores at least at some intervals after the onset of the treatment, there was no remarkable difference between the placebo and clonidine-treated groups at the end of the study. Regarding drug-related complications, a gradual decrease was seen in clonidine-associated complications, but no statistical superiority was found compared to the placebo group. Finally, the comparison of global OCD improvement revealed no remarkable difference between the two groups.
The successful outcomes of clonidine utilization for OCD management were also reported in a study by Knezevich (28) in a 22-year-old patient with refractory disease, igniting the idea of using SSRIs with clonidine augmentation therapy. In another study, Hewlett et al. (16) assessed the potential efficacy of clonidine for treating OCD. They evaluated four medications, including clonidine, clonazepam, clomipramine, and diphenhydramine (as the placebo), in a 6-week trial. The daily dose of 0.1 mg of clonidine was prescribed initially, which increased to every 2 - 4 days for four weeks until reaching the maximal dose of 1 mg. In the recent experimental study, clomipramine and clonazepam, but not clonidine, significantly improved OCD symptoms. However, clonidine was remarkably superior to diphenhydramine as the placebo (16). The outcomes of these two trials reinforced the opinion that clonidine could be considered for add-on therapy, but not as a solitary medication to control OCD.
In another study by Khanna et al. (15), the researchers evaluated the role of clonidine in improving neuroendocrine responses, raising the hypothesis that in addition to neurotransmitters, the neuroendocrine response could also play a role in OCD. Therefore, they treated their patients with clonidine and measured the serum levels of the growth hormone, cortisol, and Adrenocorticotropic hormone (ACTH). Following a significant raise in the mentioned neuroendocrine agents, and as they compared their outcomes with those of previous studies, they claimed that a significant increase in neuroendocrine components could be the etiology of OCD and proposed that clonidine can be effective in treating this disorder (15).
Franz et al. (29) in their study searched for the most efficient therapeutic approach for OCD, refractory cases in particular, and found that the use of clonidine in combination with SSRIs for two weeks could alleviate OCD symptoms; however, these effects remained uncertain and limited to some cases, necessitating further evaluations.
Contradictory results have been reported by Abdel-Ahad and Kazour (19), who assessed the efficacy of non-anti-depressant agents for treating OCD and reported the beneficial short-term effects of clonidine; however, the long-term use of this medication was not accompanied by remarkable positive effects. More recent investigations have shown that OCD presentations can exacerbate following acute phases of physical or psychological disturbances. Neuroinflammation and neuroendocrine responses, as other factors contributing to OCD, may lead to the overexpression of adrenergic biomarkers. It can be assumed that clonidine also regulates the noradrenergic and sympathetic systems, which in turn affect the levels of the growth hormone, ACTH, and cortisol, as the biomarkers responsible for acute stressful conditions, relieving compulsive symptoms eventually (16).
On the other hand, clonidine may impress the glutamatergic pathway (6). Diverse studies have shown elevated levels of glutamate, as an excitatory neurotransmitter, in the cerebrospinal fluid (CSF) of OCD patients compared to controls. This fact shows that the patients suffering from OCD struggle with a hyperarousal state that makes them present obsessions and/or compulsions (30, 31). Neuroimaging evaluations have confirmed the role of glutamate dysfunction in the pathophysiology of OCD, and volumetric assessments have revealed a structural basis for the dysfunction of fronto-subcortical circuits in OCD. These areas, including the thalamus, orbito-frontal cortex, and anterior cingulate cortex, are enriched with the glutamate neurotransmitter (32). Nevertheless, most studies have doubted the standalone role of glutamatergic dysfunction in OCD (33, 34). We hypothesized that clonidine could have an inhibitory effect on the hyperarousal state of OCD patients and might regulate patients’ intrusive symptoms. Although our patients presented promising outcomes in some of the intervals, overall outcomes in clonidine-treated patients were not significantly different compared to controls, necessitating further investigations.
5.1. Conclusions
In summary, the potential role of clonidine in the context of augmentation therapy for treating OCD has been assumed since a long time ago, but the studies conducted have presented uncertain outcomes and unanimous conclusions. Besides, no study in the literature has tried to thoroughly assess the efficacy of clonidine therapy in resolving OCD symptoms, either alone or combined with other agents. The current double-blinded controlled trial was the first study assessing the efficacy of clonidine in the treatment of OCD. Based on CGI and YBOC, two validated scales for assessing OCD severity, our results provided enough evidence to reject the null hypothesis. Also, it is noteworthy that clonidine did not cause considerable adverse effects and reported ones improved over time. Nevertheless, in order to generalize the outcomes to larger populations, further studies with larger sample sizes and more extended follow-up periods are required. Furthermore, due to restricted knowledge in this regard, we did not administer clonidine doses higher than that described in previous studies. Therefore, the administration of higher doses of clonidine may result in more prominent outcomes.
5.2. Limitations
The short follow-up period is a significant limitation of our study. In addition, there may be several unseen confounding factors that could have affected the outcomes. For instance, the duration of OCD and previous electro-convulsant therapy are among the factors that have not been included into the study checklist. Considering these limitations, further studies are recommended in this area.