1. Background
There is increasing attention toward the recognition of psychotic disorders with autoimmune etiologies, especially among first-episode psychosis patients (1). First-episode psychosis (FEP) patients have been studied widely over the past two decades. They represent a potentially promising group of patients in which early detection and interventions may prevent or reduce morbidity within the first few years (2). On the other hand, encephalitis with autoimmune causes associated with antibodies against neuronal cell-surface or synaptic proteins often accompanied with various behavioral/psychotic symptoms has received broad attention in recent years (3). Autoimmune encephalitis usually presents with psychotic symptoms before evolving into a polymorphic neurological syndrome. The prominence of timely diagnosis and proper treatment of these patients has been widely accepted among neurologists (4). It is unclear, however, if such cases are detected and treated early enough in psychiatric emergencies, particularly in the developing countries. These patients may be underdiagnosed as their main presentations are psychiatric in nature unless they develop a full range of neurological symptoms. Moreover, some cases of immunosuppressant-responsive autoimmune encephalitis have been reported to present solely with psychiatric symptoms (5). This is of paramount importance as various studies suggest early interventions for such patients is correlated with superior functioning and cognitive outcomes, less morbidity, and fewer relapses.
In the last two decades, several studies have been conducted on FEP in Iran, including our group (6). Given recent international trends in identifying cases of first-episode autoimmune psychosis (1), we adopted a modified set of criteria described in the Methods section to characterize the rate and clinical presentations of such important and potentially treatable patients.
2. Objectives
We aimed to determine the prevalence of autoimmune encephalitis in patients with FEP. We also sought to describe psychiatric and neurologic presentations in patients with FEP with and without confirmed autoimmune encephalitis.
3. Methods
This is an ongoing multidisciplinary prospective cohort study of patients with FEP referred to a major psychiatric hospital in Tehran, Iran (Roozbeh Hospital).
3.1. Patients
According to the diagnostic criteria for autoimmune psychosis proposed the by Pollak et al. (1), all patients referring to Roozbeh Hospital with FEP with the onset of symptoms over a maximum course of three months were recruited to the study. Psychosis is identified based on a score of 4 or higher in any section of the Positive and Negative Symptoms Score (PANSS) questionnaire (7) or clinical suspicion of a psychiatrist of any changes in behavior. Patients should have no history of antipsychotic medications use longer than six weeks before admission. There was no age limit for eligible patients.
According to the prevalence of autoimmune psychosis reported in previous studies (3, 8, 9), sample size was calculated to be 138 using G power. It should be noted that we were considering a subgroup of patients whose symptoms progression was within less than three months. Therefore, the prevalence of autoimmune encephalitis may be higher in our samples. There was some evidence that the prevalence of acute psychosis is higher in the developing countries. Taking these together, the sample size of this study consisted of 100 patients with first-episode psychosis meeting the eligibility criteria
3.2. Procedure
3.2.1. Initial Assessments
The patients were interviewed by a trained psychiatrist for the psychiatric examination using a series of validated psychiatric assessment questionnaires, including PANSS (7), Hamilton depression and anxiety rating scales (HAMD and HAMA) (10, 11), the WHO Quality of Life Questionnaire (WHOQOL-Bref) (12), Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (13), Young Mania Rating Scale (YMRS) (14), and Global Assessment of Functioning (GAF) (15).
All the patients underwent a comprehensive physical and neurological examination. Cognitive assessment was performed using the Montreal Cognitive Assessment (MoCA) tool (16). All the patients underwent a brain MRI and EEG study. Serum and cerebrospinal fluid were assessed for a number of metabolic, inflammatory, and autoimmune tests that will be described comprehensively in our future works. The prevalence of possible, probable, and definite autoimmune psychoses (1) is reported at the end of the study.
4. Results
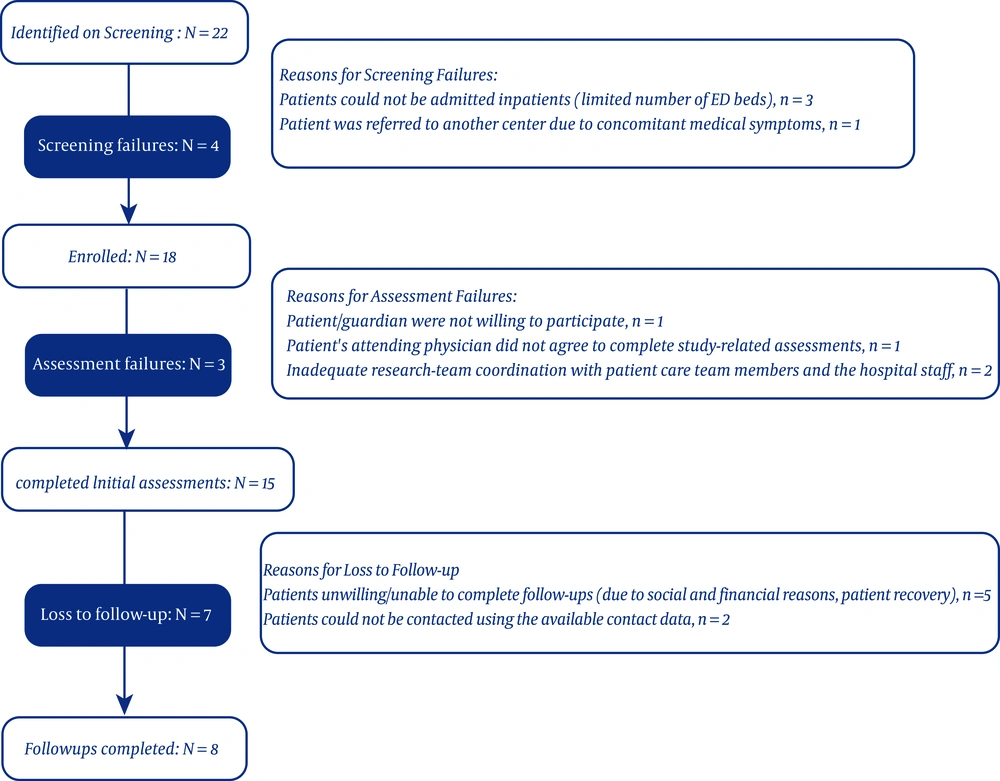
In this preliminary study, the results of the first 15 FEP patients enrolled within the last three months is presented. Twenty-two patients were identified, 18 of whom were enrolled. Initial assessments were conducted for 15 patients. The participant flow and reasons for each step failure is shown in Figure 1. None of the patients took any anti-inflammatory or immunosuppressive medications.
As seen in Table 1, seven out of 15 patients were under 20 years, and four were Afghans. Twelve patients developed prodromal symptoms, including headache, flu-like symptoms, nausea, vomiting, and changes in sleep and appetite. Nine patients presented with anxiety and mood symptoms.
| Variables | No. |
|---|---|
| Demographics | |
| Age | |
| < 20 | 7 |
| 20 - 40 | 7 |
| > 40 | 1 |
| Gender | |
| Male | 7 |
| Female | 8 |
| Marital status | |
| Single | 12 |
| Married | 3 |
| Educational status | |
| Primary | 10 |
| High school diploma | 2 |
| University | 3 |
| Occupational status | |
| Student | 7 |
| Unemployed | 5 |
| Employee | 3 |
| Ethnicity | |
| Iranian | 11 |
| Other | 4 |
| Presenting illness | |
| Onset | |
| Abrupt | 1 |
| Acute | 10 |
| Subacute | 4 |
| Course | |
| Fluctuating | 10 |
| Progressive | 3 |
| Remitting | 2 |
| Diurnal variation | |
| Yes | 5 |
| No | 10 |
| Mood and anxiety symptoms | |
| Yes | 9 |
| No | 6 |
| Negative symptoms | |
| Yes | 5 |
| No | 10 |
| Prodromal symptoms | |
| Yes | 12 |
| No | 3 |
| Mental status examination | |
| Mood | |
| Euthymic | 11 |
| Elevated | 2 |
| Depressed | 2 |
| Affect | |
| Normal | 7 |
| Restricted or blunted | 5 |
| Labile | 2 |
| Inappropriate | 1 |
| Psychomotor activity | |
| Normal | 7 |
| Retarded | 6 |
| Agitated | 2 |
| Catatonia | |
| Yes | 5 |
| No | 10 |
| Formal thought disorder | |
| Yes | 7 |
| No | 8 |
| Thought content | |
| Persecutory delusion | 13 |
| Delusion of control | 1 |
| Reference delusion | 7 |
| Grandiosity delusion | 7 |
| Misidentification | 1 |
| Nihilistic delusion | 1 |
| Suicidality | |
| Yes | 7 |
| No | 8 |
| Perception | |
| No hallucination | 4 |
| Auditory hallucination | 11 |
| Visual hallucination | 5 |
| Tactile hallucination | 1 |
All the patients had at least one type of delusion. The most frequent delusions were persecutory, reference, and grandiosity delusions. Eleven patients had experiences of auditory hallucinations, of which five had visual hallucinations simultaneously. Formal thought disorders, such as loosening of associations, incoherency, and tangentiality, were detected in seven patients.
Five patients had catatonic symptoms, mainly including mutism, posturing, and negativism.
5. Discussion
Preliminary results from our ongoing FEP project seem to be promising. There are certain sample features making our cases unique regardless of their autoimmune status, which is unclear at this stage. Out of 15 recruited patients, seven were under 20 years. Our 3-month criteria for FEP, compared to our earlier samples with six-month criteria (17-19), let us recruit a younger population compared to some other studies. This is in concert with recent proposed criteria for autoimmune psychosis as well (1). Moreover, anti N-Methyl-D-aspartate receptor (anti-NMDAR) encephalitis is more frequent in children and can present with psychosis (8). As Moreno et al. found, we are hoping that younger age of onset could lead to more cases of identifiable autoimmune psychosis (20).
Almost all of the patients had prodromal symptoms characterized by either psychiatric or physical symptoms, such as viral or neurological symptoms. This is in line with studies like Dalmau et al. (21) and Maneta et. al., who described flu-like symptoms among the factors that should prompt consideration of anti-NMDAR encephalitis in FEP (22).
We enrolled four Afghan cases, which is proportionally higher than expected. This is interesting as there is a growing body of evidence suggesting an increased risk of developing psychosis in migrant groups (23). Data on ethnic proportionality of autoimmune encephalitis is limited (24), and findings of our study may shed light on this uncovered area. In general, this group clinically resembled the common population of FEP in their presentations.
The most prevalent type of delusion was persecutory delusion, with reference delusion coming afterward. Our findings were consistent with few existing data on the theme of delusions in FEP reporting paranoid and reference delusion, the two most common delusions (25, 26). Eleven patients experienced hallucinations. This is in accordance with previous studies reporting the prevalence of hallucination in FEP at about 75% (26). Similarly, auditory hallucinations was reported to be the most prevalent type of hallucination in FEP followed by visual hallucinations (26).
However, there are some findings making our sample somewhat distinct. Five patients presented with catatonic symptoms. The prevalence of catatonia is not clear in FEP, but it is estimated to be range from 0.6 to 17% in children and adolescents (27) and 10 - 25% in mixed inpatient populations of psychiatric institutions (28, 29). In a recent case series, Averna et al. described four cases of FEP with accompanying symptoms of catatonia (27). Two of the cases were tested positive for anti-NMDAR antibodies. They were treated with risperidone without any benefits. The authors suggested psychiatrists should be aware of anti-NMDAR encephalitis in patients presenting with psychosis and dyskinesia, seizures, or catatonia to reduce diagnostic and therapeutic time delays.
Nearly half of our patients had formal thought disorders. In their study in 2019, Gibson et al. proposed psychiatric patients with anti-NMDAR encephalitis may suffer from formal thought disorders more than those with the typical presentations of FEP (30).
The prevalence and criteria for the identification of autoimmune psychosis cases have been the subject of various recent studies (1, 3). Our ongoing study similarly aims to investigate specific psychiatric profiles of such cases.
5.1. Challenges
This study has been underway in a large nationally known psychiatric hospital (Roozbeh Hospital). Some specialized services for patients with FEP, including medication and patient and family psychoeducation, are well established in this hospital. However, the referral rate has been lower than expected, given the prevalence rate of psychosis. This requires a more active recruitment strategy and education for relevant colleagues.
As a psychiatric hospital, we lack timely access to other medical teams. This, in turn, leads to practical challenges with regard to medical workups. We had to outsource some investigations; for instance, there is no MRI unit in the hospital, and specialized serum and CSF tests are not provided in the hospital laboratory. Also, patients with high fever and loss of consciousness had to be transferred to general hospitals. There are also conceptual barriers working in a psychiatric hospital as some patients and even staff consider psychiatric disorders as non-medical conditions. As such, some patients and families may feel reluctant to undergo lumbar puncture as an invasive intervention.
In this preliminary report, we provided information for the first 115 patients recruited to the study, which is obviously limited and far from our actual sample size; thus, it prevents us to draw any absolute conclusions. The most likely limitation of our study is loss to follow-up, especially in patients who became completely symptom-free after the experience of the initial episode. To address this potential barrier, we will keep our touch and educate the patients and their families. Another potential reason for patients being lost to follow-ups may be financial issues. Therefore, the costs of all diagnostic workups in addition to patient and caregiver transportation is covered by the study.
5.2. Conclusions
Studying FEP with acute onset is of paramount significance to estimate the prevalence and characteristics of autoimmune psychosis cases as a potential curable group of patients. However, there are challenges to achieve this goal, particularly working in a psychiatric hospital. Identifying specific criteria to arrive at the diagnosis of autoimmune psychosis would be a major milestone to achieve in future studies.