1. Background
Anhedonia has received special attention in diagnosing and treating psychiatric disorders for decades. Anhedonia is a transdiagnostic concept (1) that has been included in the official classification since the DSM-III and is one of the essential criteria for major depressive disorder (MDD) in the DSM-5 (2) as well as in the research domain criteria approach system (3, 4). The concept is defined as “the loss of the ability to experience pleasure” (5). Anhedonia is a hallmark of psychiatric disorders, including MDD and schizophrenia, personality disorders, eating disorders, anxiety disorders, and organic diseases (6). Furthermore, 50 to 78 percent of MDD was associated with the anhedonia symptom in the UK (7). In previous research, anhedonia was the only dimension that could predict a longer recovery time and poor treatment outcome (6). Therefore, the concept is significant as a specific prognostic feature and therapeutic target for people with recurrent depression.
In recent years, the assessment of anhedonia has received renewed attention. Updated conceptualization suggests that anhedonia includes desire, effort, subjective pleasure, and cognitive components (e.g., the capability to predict and anticipate award) (1, 8, 9). Also, research shows that approximately 45% of the studies that measured anhedonia did not define the concept (10). These dilemmas in anhedonia conceptualization have made the measurement of anhedonia difficult and inaccurate, leading to a lack of good tools to address various aspects of the “pleasure response” (8).
The most widely used tools for evaluating anhedonia include the Snaith-Hamilton Pleasure Scale (SHAPS) (11), the Faust-Clark Pleasure Capacity Scale (FCPS) (12), the Renewal Scale, Chapman Physical Anhedonia (CPAS), and Chapman Social Anhedonia Scale (CSAS) (13, 14). Although most of these tools have been used in previous research, they have some limitations. These scales are sometimes time-consuming, culturally biased, and outdated. They may also have poor differential validity and the fact that existing tools must fully reflect all components of reward processing to adequately assess anhedonia. As a result, the lack of any of these dimensions can underestimate the evaluation of the pleasure experience, so that this evaluation can cover the various components of the structure (5, 15).
Finally, Rizvi (15) developed a Dimensional Anhedonia Rating Scale (DARS), unlike previous scales that focus on the specific components of anhedonia (9, 16-18). It has an integrated approach to evaluating anhedonia and evaluates various characteristics, such as interest, motivation, virtual activities, and enjoyable consumption across different areas, namely entertainment, food and drink, social activities, and sensory experiences. All four dimensions in the DARS are essential and must be answered thoroughly. Confirming this point, Lambert suggests that DARS is the only scale that has so far been evaluated validly in an MDD (major depression disorder) sample that assesses the various aspects of anhedonia (1).
The DARS questionnaire was designed to investigate as many aspects of reward processing as possible where anhedonia may be based on irregularities (8). DARS considers interest, effort, motivation, and satisfaction across hedonic domains (15). The persons completing the questionnaire provide examples of their favorites in the four subscales: hobby (hobby/pastimes), food/drinks, spending time with friends (social activities), and sensory experiences (15). DARS has more predictive power than the best pre-existing alternative questionnaire (SHAPS) and helps diagnose the subtypes of depression (such as recurrent depression) (9). The questionnaire requires respondents to express some of the activities they perceive as satisfying (e.g., gardening and playing the guitar under hobbies/past times), which may include some of the issues related to age differences in what people might find rewarding (adolescents vs. adults vs. elderly) (5, 15, 19).
Previous studies have reported excellent internal consistency of DARS and a significant correlation between DARS and the severity of depression (9, 15, 20, 21). It also supports the idea that anhedonia is an overlapping but distinct structure with depression (5). Also, it explains the higher depression variance than SHAPS. Clinically determining MDD subtypes and the relevance of such clinical aspects to neurobiology is crucial for improving treatment selection strategies (22).
2. Objectives
Among the questionnaires that measure anhedonia, only the SHAPS has been standardized in the Iranian population (23). Because of the advantages of DARS for measuring anhedonia in the Iranian population and good psychometric indices of the DARS in its original version and other studies, this study aimed to translate DARS into Persian (Farsi) and determine its validity and reliability in a group of different patients (9). In addition, because previous studies did not examine the stability of the questionnaire, one of the objectives of this study was to investigate the stability of anhedonia.
3. Methods
3.1. Participants
The research utilizes a cross-sectional descriptive and factor analysis to inquire about the validity and reliability of DARS in the Iranian population. First, the scale was translated into Persian (Farsi) by three clinical psychologists, one psychiatrist, and an English language expert. The scale was reverse-translated to English following an initial English to Persian translation. No items were omitted or changed. Data were collected online through social media. In such a way that after distributing the announcement in cyberspace (Instagram, Telegram, WhatsApp), the form of the handouts was sent to the participants. The inclusion criteria were having Iranian nationality, both genders, age (age range between 18 and 55 years) under sixty, and reading and writing ability. The exclusion criteria were those who did not complete the questionnaire. A clinical psychologist handed them a consent form after the subjects were informed about the study objectives and procedures. They were included in the study following their written informed consent. All participants were selected using the convenience sampling method. Previous research mentioned the sample size needed for exploratory and confirmatory factor analysis was between 200 and 500 (24). The sample size for the current study was 474. In addition, 44 individuals participated in the study to assess the test-retest reliability. Test-retest was done in 3-week intervals.
3.2. Statistical Analysis
Descriptive and inferential statistical methods were employed to calculate central indicators and dispersion. The exploratory factor analysis method was used to determine the subscales of the questionnaire. SPSS version 24 was used for the statistical calculations. In addition, Cronbach’s alpha (for reliability) and test-retest stability were used. Also, discriminant validity and inter-correlation of subscales were used. MANOVA was used to investigate gender differences in DARS and its subtypes. The demographic data were analyzed using mean, standard deviation (SD) descriptive statistics to address the study’s first objective. Prior to the statistical analysis, the outlier’s data were checked. A visual check confirmed normal distribution of the data. Reliability, which relates to the consistency and stability, has been assessed with the internal consistency of test and retest (i.e., Cronbach’s α). Confirmatory factor analysis (CFA) was conducted using AMOS software. Data were divided into two halves, and CFA and EFA were done in separate data halves. The study was conducted after obtaining the code of ethics from the University of Social Welfare and Rehabilitation Sciences (Approval Code: IR.USWR.REC.1400.102).
3.3. Measures
Dimensional anhedonia rating scale: The DARS is a self-reported questionnaire that measures four domains of hedonic responses (5). The scale has 17 items rated by a Likert scale from 1 (not at all) to 5 (very much). The factor structure of DARS revealed four components related to the four domains of hedonic responses (hobbies, sensory experience, social activities, and food/drink). According to Rizvi’s study (5), the DARS was internally consistent (Cronbach’s α = 0.75 - 0.92).
3.3.1. Beck’s Depression Inventory (2nd Ed.)
This scale is the revised version of Beck’s depression inventory, which was composed to measure an individual’s depression level (25). It includes 21 items. The four choices of each question are scored in a four-level spectrum from 0 to 3. Therefore, the total score of the questionnaire is in the range of 0 to 63. The internal consistency of the inventory is reported as 0.91 (25). The psychometric properties of the inventory in Iran are reported as Cronbach’s alpha coefficient of 0.91, a correlation coefficient between two halves of 0.89, and a one-week retest coefficient of 0.94. The DARS also demonstrated good convergent and divergent validity with SHAPS (26, 27).
4. Results
The statistical population of the current study was 474. The age range was between 18 and 58 years (M = 29.5, SD = 8.49). Study demographics showed 82 (17.2%) males and 325 (82.8%) females, among which 164 (34.5%) were married, 225 (47.9%) were single, and 83 (17.5%) divorced. The importance of managing missing data is the reason for the large sample size of the present research. Considering that missing data reduces the sample size and small sample size is also problematic in the exploratory/confirmatory factor analysis per se; hence, the missing data replacement method was used with the variable mean for managing the missing data. Table 1 represents the descriptive statistics for any subscale and the entire scale. The DARS scores were normally distributed, skewness = -0.63 (SE = 0.18), kurtosis = 0.59 (SE = 0.37), with no evidence of univariate or multivariate outliers. The DARS total score was 47.61 ± 13.65.
| Variables | Scale Mean If Item Deleted | Scale Variance If Item Deleted | Corrected Item-Total Correlation | Squared Multiple Correlation | Cronbach’s Alpha If Item Deleted |
|---|---|---|---|---|---|
| DARS1 | 48.93 | 57.32 | 0.40 | 0.54 | 0.82 |
| DARS2 | 49.78 | 56.84 | 0.31 | 0.30 | 0.83 |
| DARS3 | 49.27 | 56.82 | 0.39 | 0.30 | 0.82 |
| DARS4 | 48.84 | 57.80 | 0.38 | 0.48 | 0.83 |
| DARS5 | 50.01 | 53.69 | 0.36 | 0.34 | 0.83 |
| DARS6 | 48.82 | 57.18 | 0.46 | 0.36 | 0.82 |
| DARS7 | 49.71 | 54.16 | 0.50 | 0.43 | 0.82 |
| DARS8 | 49.46 | 57.19 | 0.31 | 0.16 | 0.83 |
| DARS9 | 49.10 | 55.18 | 0.51 | 0.42 | 0.82 |
| DARS10 | 49.56 | 55.78 | 0.35 | 0.36 | 0.83 |
| DARS11 | 50.07 | 52.80 | 0.51 | 0.48 | 0.82 |
| DARS12 | 49.65 | 54.22 | 0.47 | 0.48 | 0.82 |
| DARS13 | 49.44 | 53.54 | 0.54 | 0.47 | 0.82 |
| DARS14 | 49.31 | 53.77 | 0.52 | 0.49 | 0.82 |
| DARS15 | 48.98 | 55.89 | 0.46 | 0.44 | 0.82 |
| DARS16 | 49.10 | 55.80 | 0.48 | 0.49 | 0.82 |
| DARS17 | 49.75 | 53.37 | 0.54 | 0.55 | 0.82 |
According to Table 1, the internal consistencies of the variables were acceptable. Cronbach’s α indicated good internal reliability, α = 0.84 for the total scale.
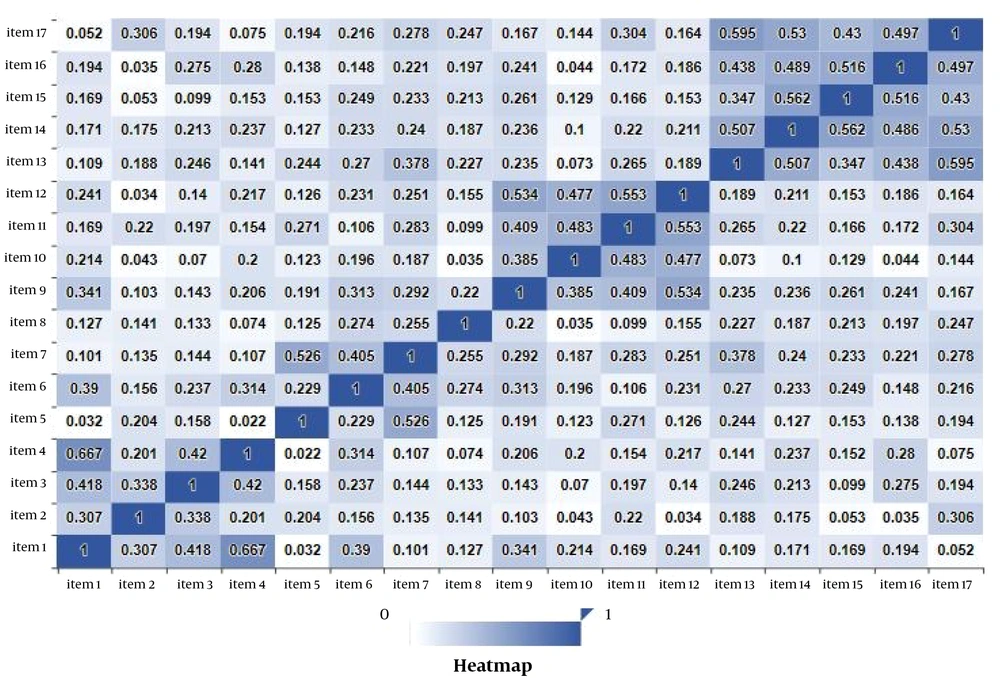
Test-retest reliability was done for the total score and the four components of the scale to calculate the stability of the DARS. The interval of test-retest was three weeks. The analysis findings show good stability across the DARS and all four components with significant ICC between the first and second tests. The obtained ICC scores included total DARS ICC = 0.88; Pastimes/Hobbies, ICC = 0.84; Foods/Drinks, ICC = 0.86; Social activities, ICC = 0.76; and Sensory experience, ICC = 0.78. Figure 1 represents the correlations of the subscale together with the total score of DARS for determining the construct validity. According to Table 1, all the subscales significantly (P < 0.01) correlated together and to each other and significantly correlated with the total score of DARS. In addition, the total DARS score negatively correlated to BDI (-0.395, P < 0.000), which showed medium and acceptable congruent validity. Also, independent t-test results showed no statistically significant differences in terms of gender in DARS and its subscale in females and males [(f = 0.365) = 0.548, Sig = 0.09].
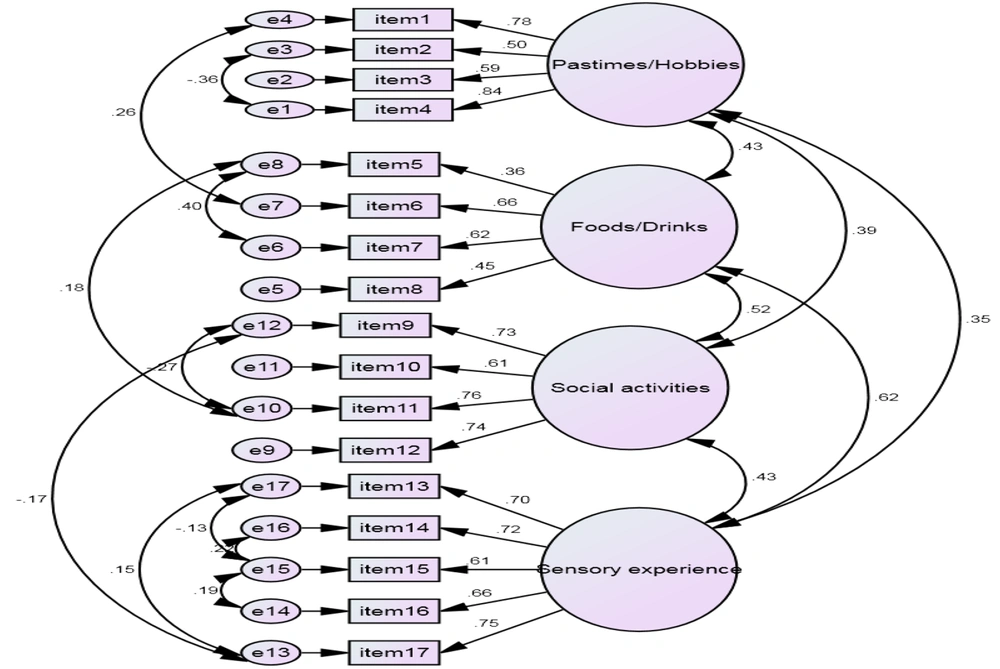
As suggested by Harrington (28), data were randomly divided into two parts and EFA was implemented with principal components analysis method for the first part and CFA for the second part to evaluate the construct validity obtained in EFA of the first part. Before exploratory factor analysis, KMO and Bartlett’s sphericity tests were used to examine the acceptability of the sample size. The results from the KMO Measure (0.82) and Bartlett’s Sphericity test (chi-square = 2033.93; Df = 45; P < 0.000) were indicative of the suitability of the data for factor analysis. Exploratory factor analysis (EFA) was conducted on the data. After rotation (using the quatrimax method), the principal component analysis indicated that four factors could explain 58% of the variance. As shown in Table 2, the factor load of the items is between 0.50 and 0.84. AMOS software version 20 was used to confirm the factorial structure of variables produced by the CFA. Results show a 4-factor structural model that was hypothesized for the questionnaire. The results of the model fit indices are presented in Table 3, confirming the suitability of the model. Based on the results of Table 3, the 4-factor model of the DARS has a good fit. The model modification indices were examined to improve the model fit.
| Items | Factors | |||
|---|---|---|---|---|
| Pastimes/Hobbies | Foods/Drinks | Social Activities | Sensory Experience | |
| Items 1 | 0.846 | |||
| Items 2 | 0.556 | |||
| Items 3 | 0.503 | |||
| Items 4 | 0.807 | |||
| Items 5 | 0.686 | |||
| Items 6 | 0.605 | |||
| Items 7 | 0.789 | |||
| Items 8 | 0.507 | |||
| Items 9 | 0.629 | |||
| Items 10 | 0.776 | |||
| Items 11 | 0.776 | |||
| Items 12 | 0.803 | |||
| Items 13 | 0.661 | |||
| Items 14 | 0.789 | |||
| Items 15 | 0.735 | |||
| Items 16 | 0.775 | |||
| Items 17 | 0.742 | |||
The fit indices observed in Table 3 and their standard parameters are depicted in Figure 2. All items exhibited significant factor loadings, as indicated in Figure 2 and Tables 2 and 3.
| Variables | X2 (df) | df | CFI | GFI | RMSEA | NFI | TLI |
|---|---|---|---|---|---|---|---|
| Model | 6353.15 | 2.74 | 0.93 | 0.92 | 0.08 | 0.87 | 0.91 |
Abbreviations: df, degree of freedom; CFI, comparative fit index; GFI, goodness of fit index; RMSEA, root-mean-square error of approximation; NFI, normal fit index; TLI, Tucker-Lewis’s index.
5. Discussion
The present study attempted to investigate the dimensional structure of DARS in the general population. Study results have shown that the Persian DARS is a reliable and valid self-report scale. The internal consistency of DARS and its subscales is good. The Cronbach’s alpha coefficient (overall 0.84 and 0.82 - 0.87 for subscales) was comparable to the previous research (overall Cronbach’s alpha coefficient = 0.84 and 0.75 - 0.99 for subscales) (5, 9, 20, 21). In addition, for the social activities factor, the internal consistency was higher (0.91) than in any of the three samples in the original version (0.83 - 0.88) (9). Furthermore, the test-retest coefficients (0.77) further confirmed the stability of the entire scale and its subscales. Next, Cronbach’s alpha coefficient showed sufficient value in the Iranian DARS version and its subscales regarding internal consistency. The total score and the subscale values of 0.54 to 0.75 were obtained regarding the retest reliability. Only for “food/drink,” the correlation coefficient was relatively low. However, it can be said that there was sufficient test-retest reliability when examined comprehensively.
In addition, the correlation observed for the BDI and DARS was medium convergent. The results showed an important positive relationship between DARS and depression. The results confirm that the convergent validity of the DARS was good and consistent with previous research (5, 20). According to Rizvi’s (5) study, DARS distinguished MDD vs. non-depressive healthy participants and treatment-resistant depression patients in the MDD group in contrast with the SHAPS. In addition, DARS explains 14% of the variance of depression in the model predicting the severity of depression (15).
On the other hand, the moderate correlation between DARS and depression indicates that the DARS questionnaire is a distinct construct that measures different dimensions of psychopathological symptoms that are not considered in the DSM-V criteria. Also, the other dimensions of psychopathological symptoms do not overlap with the official symptoms of depression. Regarding group and gender, the independent t-test showed no significant difference between female and male groups in the concurrent validity. This study aimed to develop a Persian version of DARS and confirm its reliability and validity. As a result of the principal component analysis, when the analysis was performed with the principal component fixed, the same result as the original DARS was obtained, and the load was also sufficient. Therefore, the factor validity of the Persian version of DARS was confirmed.
As for construct validity, it was found that the Persian version of DARS is a valid measure. Results of EFA to determine the construct validity of DARS showed four extracted factors. These four factors explained nearly 58% of the variance (relatively similar to previous research 59.2% in the original version and 60.4% in the Spanish scale (9, 15). However, the percentages of variance explained for each subscale were more similar to the original than the Spanish scale. Also, the percentage of variance explained in the Persian version was between 8% to 30% (similar to the original version), while the range is more uniform in the Spanish version. Unlike the original version, sensory experiences were the most critical factor, and Foods/Drinks had an almost negligible effect on the total score (9).
Finally, the limitations of this study should be considered. In this study, we used an online survey to examine the reliability and validity of the Persian version of DARS in community samples. First, the limits of the online survey will be described. Although online surveys are highly convenient and practical, it has been pointed out that sample bias is likely to occur and prevents access to the complete clinical features of the participants. One of the strengths of this study is that the questionnaire was localized to 474 Iranian nationals and was not specific to a particular group; hence, it applies to all target populations in Iran. In addition, it is necessary to survey the clinical group to examine the reliability and validity of the community sample only. At the same time, the usefulness of the Persian version of DARS as a psychological scale could increase if the cut-off value can be set like SHAPS through a comparison between the normal group and the clinical group.
5.1. Conclusions
Previous anhedonia questionnaires measured only self-fulfilling pleasure; however, the DARS can measure various interest, motivation, effort, and self-fulfilling pleasure components. We examined the reliability and validity of the Persian version of DARS in the community sample. For reliability, internal consistency and retest reliability were examined, and for validity, factor validity and construct validity were scrutinized. As a result, the Persian version of DARS showed sufficient internal consistency, retest reliability, and factor validity and showed certain construct validity. Although further studies are needed on reliability, validity, and usefulness in the clinical group, the Persian version of DARS has a certain degree of usefulness as a multifaceted measure of anhedonia.