1. Background
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with an increasing prevalence over the past few years, accompanied by increased interest and investment in research on ASD (1, 2). The heterogeneous manifestations suggested its labeling as a spectrum disorder; however, its major symptoms are decreased social communication, restrictive interests, and repetitive behavior patterns (3). Most patients with ASD have difficulty recognizing facial expressions, the ability that others gain shortly after birth and enables them to understand the emotions of others and interact with them. Misunderstanding facial expressions results in delay and deviance in developing social, communicative, and cognitive skills in children with ASD since the first years of life (4).
Although the symptoms are usually diagnosed since childhood, treatment of ASD is still a major challenge, and the symptoms may continue until adolescence or even adulthood. Considering the adverse effects of most medical interventions suggested for ASD and their questioned benefit for treating patients' symptoms (5), behavioral and psycho-educational management strategies have been suggested and are currently considered the gold-standard treatment of ASD (6). Nonetheless, behavioral therapies have disadvantages, such as the need for continuance, optimum effectiveness only when initiated early in life, and costs. Some families search for alternative methods, often without medical supervision (7). Therefore, there is still a need for more effective treatment with lower cost and shorter duration to apply to families.
One alternative could be transcranial electrical stimulation (tES), a non-invasive and painless method previously confined to research settings but currently used in everyday clinical practice for neuropsychiatric disorders. Electrical stimulation of the brain was initially used for functional mapping of the human brain with the ability to assess the perceptual or behavioral function of that brain region (8, 9). This non-invasive stimulation can be given by either transcranial direct current stimulation (tDCS) or transcranial alternating current stimulation (tACS). In tDCS, the application of low-intensity (weak) and direct electric current is limited to the cortex, and the modification of electrode size can increase its low spatial focality to different areas of the cerebral cortex through the scalp influence on the neuron excitability (10), which can facilitate or inhibit the activity of the nerves (11, 12). The after-effects of the stimulation, modulated by glutamatergic synapses, can result in long-term potentiation and depression-like mechanisms; however, the exact mechanism of action is still under investigation (13).
Besides the effect of tDCS on symptoms of depression, psychosis, and schizophrenia (14), it has been postulated that the induced neuroplastic changes can have a beneficial effect on inhibitory control, working memory in attention-deficit hyperactivity disorder (ADHD) (15), dyslexia (16), and cerebral palsy (17). It has also been shown to improve the social-cognitive performance of healthy subjects (18). Favorable results have also been reported by randomized clinical trials (RCTs) and pre-post studies of active tDCS stimulation in patients with ASD (19-23). A review study determined significant improvement in post-stimulation assessment, more prominent than the sham, confirming the efficacy of tDCS stimulation for ASD (13). Also, most studies have reported no or mild adverse effects for this treatment, which confirms its safety (13, 19, 20, 24). The clinical improvement was also maintained until six months (25). Longer follow-ups (e.g., one year) have been only reported in case reports (26), and the long-term effects of tDCS stimulation for ASD should be confirmed in further studies.
The studies available on the efficacy of tDCS stimulation for ASD have included different subjects; some have evaluated the adult population (22, 23, 26), and others have evaluated children with specific features of ASD, such as minimally verbal children (21). As far as we know, only one study has evaluated the efficacy of tDCS stimulation in facial emotion recognition (FER) of patients with ASD, including adults only (26). Considering the significance of FER in symptoms of ASD addressed above, it is important to evaluate the effect of tDCS stimulation on this disease feature in the pediatric population. We also evaluated the autism treatment evaluation checklist (ATEC), the most utilized in similar studies (17, 19, 20).
2. Objectives
In this study, we aimed to investigate the effectiveness of tDCS stimulation of the left dorsolateral prefrontal cortex (DLPFC) in FER and ATEC in children with ASD to determine whether this non-invasive treatment can improve the clinical symptoms of these patients.
3. Methods
This quasi-experimental study was performed by a pretest-posttest design with intervention and control groups. The group variable, with two levels (tDCS and control), was the between-subject variable, and the test time (assessment time), with two levels (pretest and posttest), was the within-subject variable.
3.1. Participants
Considering the male dominancy of ASD and its high prevalence at school age, we selected a boy school, Edalat School, Tehran, Iran, for sampling in the academic year 2020 - 2021. This school is under the supervision of the General Department of Exceptional Education in Tehran. The researcher referred to the school and, after coordination with school officials, selected the eligible participants based on the following criteria: Age of 6 - 17 years, diagnosis of ASD by a specialist, no history of susceptibility or suspected epilepsy, consent of parents and school officials for their participation, and not participating in another training program simultaneously with this study. The researcher informed the parents and teachers about the research purpose and methods through group lectures and asked both parents to read and sign written informed consent; one copy was given to the school, and one copy was kept with the researcher.
The sample size was calculated as 24 in total, using G*Power 3.1.9.2. In the test family of F tests and statistical tests of within-between interactions, the input parameters were an effect size of 0.4, an α error probability of 0.05, and a power of 0.95 for two groups and two dependent variables. The eligible participants were enrolled in the study, based on this sample size, using a convenience sampling method.
The selected children were randomly assigned to the experimental and control groups. The average age, intelligence quotient (IQ), and ASD severity of the two groups were similar (Table 1). The IQ scores were measured using Raven's standard progressive matrices for children, and the disease severity was measured using the Gilliam Autism Rating Scale (GARS-3) (Table 1).
| Measure | Experimental Group | Control Group | t | df | P Value |
|---|---|---|---|---|---|
| Age (y) | 9 ± 2.36 | 9.81 ± 2.4 | -0.8 | 20 | 0.74 |
| Intelligence quotient | 85.27 ± 9.94 | 83.72 ± 10.28 | 0.35 | 20 | 0.43 |
| Severity of autism spectrum disorder | 97.36 ± 8.2 | 93.54 ± 8.75 | 1.05 | 20 | 0.3 |
a Values are expressed as mean ± SD unless otherwise indicated.
3.2. Instruments
The following tests were used to assess the dependent variables:
1. The emotion recognition task, designed in 2009 (27), consists of 44 face pictures that show six basic emotions. The emotional facial pictures depicted men/women with low/high intensity extracted from the NimStim set of facial expressions database. The researcher showed the pictures to the participant and asked them to select the depicted emotion from the pre-determined list of emotions, including anger, happiness, sadness, disgust, fear, and surprise. The reliability of this instrument has been confirmed in developing children using the split-half method with Spearman-Brown's coefficient of 0.857 and Guttman's coefficient of 0.852. Its validity was also confirmed by its correlation with the theory of mind, amounting to 0.43, significant at P < 0.05 (28).
2. The ATEC, designed by Rimland and Edelson (29), has 52 items and four subscales to evaluate the effect of interventions on autism. This instrument has enough sensitivity to assess any change in the child's situation. Its reliability was confirmed with values between 0.81 and 0.92 for the subscales and 0.94 for the total scale. Its validity was proven by its correlation with similar scales at 0.79.
3.3. Intervention
The tDCS was applied to stimulate the subject's brain (on the DLPFC area). The apparatus used was the STARSTIM model tDCS, manufactured by Neuroelectrics Company in Spain. For the experimental group, the anodal method was performed for 15 minutes with 2 mA intensity in 10 sessions with a 72-hour interval between the sessions. The control group received 20-second sham stimulation by placing the electrodes in the same positions as the active stimulation. This caused the control group participants to experience an initial itching sensation of tDCS without receiving the active stimulation current.
If the participant could not complete the ten sessions or did not tolerate the intervention, he was excluded from the study. Also, if any side effect occurred, such as headache, the intervention was discontinued, and the participant was excluded from the study. According to these exclusion criteria, two participants were excluded from the study, one because of the parental report of headache and another because of a low tolerance threshold.
3.4. Statistical Analysis
Since there was one between-subject independent variable (group: Experimental and control), one within-subject independent variable (pretest, posttest), and two dependent variables (FER scores and ATEC, measured by ratio and interval scales), two separate mixed ANOVA tests were performed using SPSS software, version 25. The significance level was set at α < 0.05, and the effect size was calculated by Eta squared. For numeric variables, first, the assumption of normal distribution of the scores was tested by the Kolmogorov-Smirnov test, and the assumption of homogeneity of variance was tested with Levine's test; the statistical test was selected according to the results of these tests. There was no need to perform Mauchly's test of sphericity in either of the dependent variables because the within-subject variable in this study had only two levels (pre and post-test); thus, the sphericity assumption was met.
4. Results
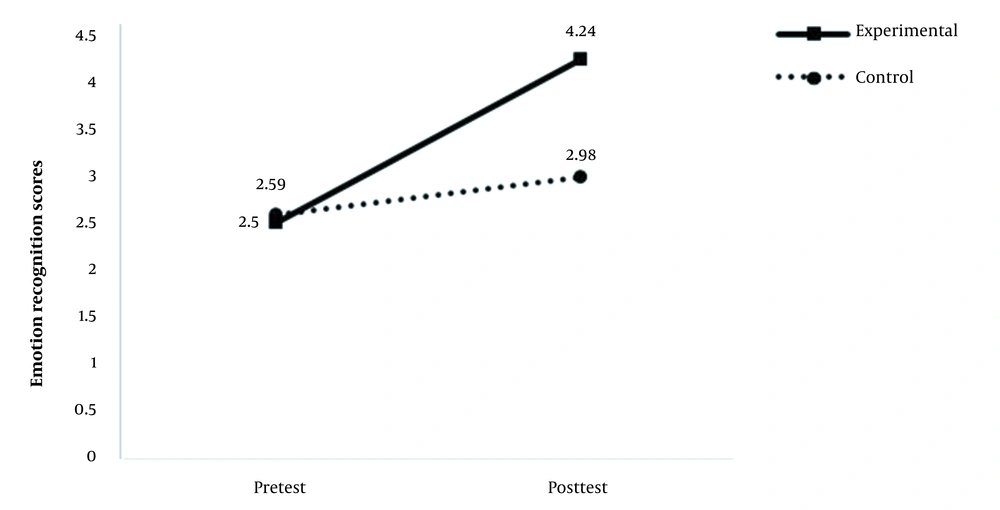
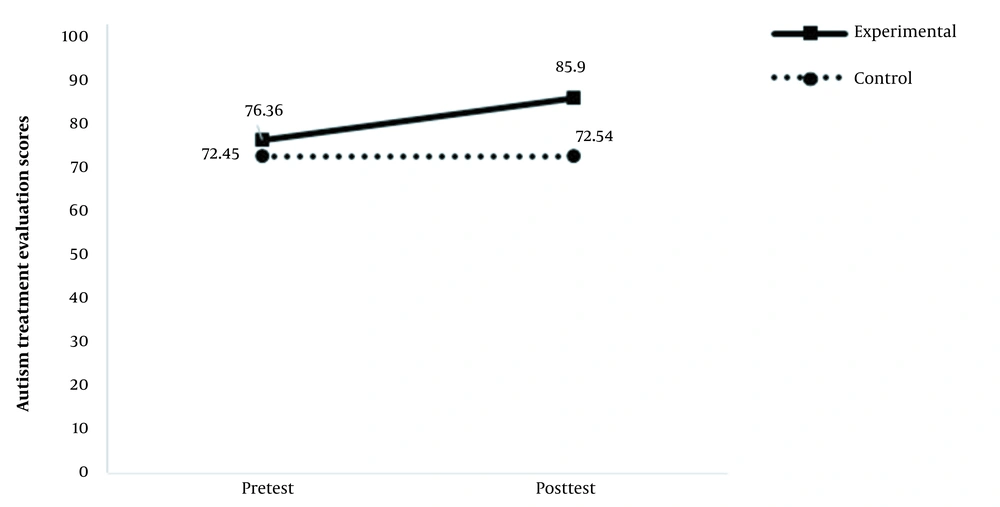
A total of 10 participants completed the study as the experimental group and 11 as the control group. The mean scores of the two instruments in the pre and post-test stages are shown in Table 2.
| Measure | Experimental Group | Control Group | ||
|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | |
| Emotion recognition task | 2.5 ± 0.45 | 4.24 ± 0.73 | 2.59 ± 0.63 | 2.98 ± 0.58 |
| Autism treatment evaluation checklist | 76.36 ± 14.1 | 85.9 ± 16.2 | 72.45 ± 10.2 | 72.54 ± 9.7 |
The Kolmogorov-Smirnov test showed that the Z values were not significant for any of the emotion recognition scores (Kolmogorov-Smirnov Z = 0.7, P = 0.71 for pretest scores and Kolmogorov-Smirnov Z = 0.62, P = 0.83 for posttest scores); therefore, the assumption of normal distribution was met. For the assumption of homogeneity of variance, the results of Levine's test showed that this assumption was met (F(1, 20) = 1.93, P = 0.18 for pretest scores and F(1, 20) = 1.82, P = 0.19 for posttest scores). Therefore, mixed ANOVA was performed to investigate the effect of tDCS on the emotion recognition scores of boys with ASD, as presented in Table 3.
| Source | Sum of Squares | df | Mean Square | F | P Value | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Within-subject | ||||||
| Test time | 12.49 | 1 | 12.49 | 50.23 | 0.001 | 0.71 |
| Test time* group | 4.93 | 1 | 4.93 | 19.82 | 0.001 | 0.5 |
| Error | 4.97 | 20 | 0.25 | |||
| Between-subject | ||||||
| Group | 3.77 | 1 | 3.77 | 7.65 | 0.05 | 0.27 |
| Error | 9.87 | 20 | 0.49 |
According to the results of mixed ANOVA (Table 3), the interactive effect of group and test time on the emotion recognition scores was significant. The partial eta squared in Table 3 shows that the independent variable could explain 50% of the dependent variable variance. Figure 1 provides a better illustration of this interactive effect; as shown, the scores of both groups were approximately equal in the pretest phase, but in the posttest phase, the scores of the experimental group increased significantly, while the scores of the control group showed only a slight increase.
The Kolmogorov-Smirnov test confirmed the assumption of normal distribution of the ATEC scores since the Z values were not significant for any of the scores (Kolmogorov-Smirnov Z = 0.77, P = 0.58 for pretest scores and Kolmogorov-Smirnov Z = 0.6, P = 0.86 for posttest scores). The assumption of homogeneity of variance was also met, based on Levine's test results (F(1, 20) = 1.26, P = 0.27 for pretest scores and F(1, 20) = 1.3, P = 0.26 for posttest scores). Therefore, mixed ANOVA was used, the results of which are presented in Table 4.
| Source | Sum of Squares | df | Mean Square | F | P Value | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Within-subject | ||||||
| Test time | 255 | 1 | 255 | 9.8 | 0.005 | 0.33 |
| Test time* group | 245 | 1 | 245 | 9.44 | 0.01 | 0.32 |
| Error | 520 | 20 | 26.04 | |||
| Between- subject | ||||||
| Group | 820 | 1 | 820 | 2.7 | 0.11 | 0.12 |
| Error | 6098 | 20 | 304 |
According to the results presented in this table, the interactive effect of group and test time on ATEC scores was significant at 0.01 level. The partial eta squared in Table 4 shows that the independent variable could explain 32% of the dependent variable variance. Figure 2 provides a better illustration of this interactive effect, which indicates no significant change in the pre- and post-test phases in the control group, while the scores of the experimental group increased significantly in the post-test phase compared to the pretest phase.
5. Discussion
The present study confirmed that ten sessions of tDCS, with the mentioned details, could improve the clinical symptoms of school-aged boys with ASD. We hypothesized such an effect based on the previous evidence on the effect of tDCS on different aspects of ASD, such as imitation-inhibition and perspective-taking (30), balance (31), and social functioning (32). To evaluate the effect of treatment on participants' clinical symptoms, we used the most commonly used instrument to evaluate the effect of ASD treatment, ATEC (33). In addition, FER, critical to many aspects of social communication, is impaired in most patients with ASD (34, 35); therefore, enhancing FER deficit can be an effective treatment strategy for improving social communication in such patients (36). The mean score of the emotion recognition task in the present research (about 2.5 in both groups) showed a FER deficit in school-aged children with ASD, which aligns with previous research, indicating the significance of FER deficit in patients with ASD (37, 38). The post-test results in the present study determined the significant effect of treatment on this variable.
Few studies are available on the effect of tDCS on FER of patients with ASD, mainly on a limited sample size. In one study on seven adult patients with ASD, the researchers showed improved performance on the empathy quotient by anodal tDCS of the right temporoparietal junction (26). The intensity used in this study was similar to ours (2 mA), but they showed no significant effect on FER, which contradicts our results. In another study on six adults with ASD, they determined that the effect of tDCS (with the same characteristics as the previous study) resulted in the appearance of FER eight minutes after the stimulation initiation, which also helped to improve verbal fluency compared with sham (39). In another study, the authors showed improved empathy and FER in adults with ASD, following tDCS (40), which is consistent with the results of the present study. Also, the orbitofrontal cortex anodal tDCS (two sessions) enhanced FER in healthy adults more than in the sham group (41). Another study also showed that anodal tDCS applied over the left temporal cortex increased the performance of healthy subjects to FER (42). These results align with the present study, considering the effectiveness of tDCS in FER deficit of patients with ASD, although the details of the stimulation, like brain regions selected for the anodal and cathodal stimulation and the instrument used for FER measurement, differed in the studies.
Others have also shown that anodal tDCS of the right temporoparietal junction could help diagnose FER deficits in patients with ASD, used to elucidate the nature and distribution of underlying neurophysiological processes (9). It has been suggested that the stimulation of these brain regions in patients with ASD using tDCS helps patients in the recognition and processing of facial emotions (43), confirmed by electroencephalography (44, 45); however, more studies are required to understand the exact mechanism of action for this effect.
Another variable measured in the present study was ATEC, which has been frequently used for evaluating the effectiveness of treatment strategies for ASD on clinical symptoms (13, 33). The present study showed a favorable effect of this intervention on ATEC, which aligns with previous studies' results (19, 20, 24). In a study on 20 children aged 9 - 14 years, 20 sessions of 1 or 1.5 mA (for ≤ 10 and > 11 years, respectively) anodal tDCS with the anode placed in F3 and cathode in the occipital region (right cerebellum) significantly improved ATEC in the intervention (but not sham) group (46); these results are in line with the present study. Also, in a study on 20 boys with ASD, aged 5 - 9 years, 20 minutes of anodal tDCS placed at left DLPFC could decrease the total score of ATEC and its health/behavioral problems (19). In another study, the researchers showed that the effect of tDCS on ATEC (two domains of social and health/behavioral problems) started 24 hours after the stimulation (20). Other researchers investigating 50 patients aged 4 - 14 also showed that ten sessions of 1 mA anodal tDCS (each for 20 min) on DLPFC significantly reduced ATEC scores, including total score, sociability, health, physical, and behavior subscores (45). These results align with the present study, considering the effectiveness of tDCS in ATEC in children with ASD. However, the details of the stimulation, like brain regions selected for the anodal and cathodal stimulation, the intensity, and duration of stimulation differed among the studies.
The main strength of the present study was the evaluation of the effect of this novel treatment on an important aspect of ASD that had not been investigated comprehensively before as far as concerned. However, this study had some limitations. One of the limitations was related to the study's sample size and dropouts during the study period. Although the sample was selected based on the calculated sample size, larger groups could help increase the reliability of the results. Furthermore, we selected boys from one school in Tehran; therefore, the results cannot be generalized to all pediatric patients with ASD. Another limitation was related to the inclusion of participants in the study by the non-randomized method, which increased the risk of the effect of confounders on the results. The last but not least limitation was related to the lack of follow-up in the present study; the post-test results were based on the outcomes measured in the final session of the intervention. Accordingly, we cannot comment on the long-term effects of this treatment strategy on this group of patients.
5.1. Conclusions
According to the results of the present study, ten sessions of tDCS (with an intensity of 2 mA) could improve the FER deficit in school-aged boys with ASD. This parameter, FER, is critical for social communications, the main deficit in patients with ASD, and its improvement can enhance the patients' social relations. A few studies have addressed the efficacy of this novel treatment on this important component, reporting controversial results. Investigating this issue in future studies on a larger and broader sample of patients with a longer follow-up is necessary. Another important result obtained by the present study was related to the improved ATEC score after tDCS, shown in previous studies without controversy about its effectiveness. Considering the effectiveness of this treatment, it is worth investigating its safety and efficacy in future studies to include this non-invasive intervention in the routine treatment protocol of patients with ASD.