1. Background
Depression, as one of the most prevalent disorders (1), is the main cause of disability worldwide (2). People are much more likely to develop depression if they already have an anxiety disorder. The mechanisms accountable for these brain disorders are mostly connected to the reduction of monoamines’ levels (noradrenaline, serotonin, and dopamine) in some central nervous system (CNS) structures, including the prefrontal cortex and hippocampus (3). Depression is usually treated with medications, psychiatric methods, or a combination of both treatment methods. However, it has been seen that many patients with this kind of disorder will reject pharmacological and psychiatric medications. Thus, the main influential treatment for depression is electroconvulsive therapy (ECT) (4, 5). ECT may play a role in people with comorbid depression and anxiety. For the antidepressant activity of electroconvulsive seizures (ECSs), as an analog of ECT that causes general seizures in laboratory animals (6, 7) and could antagonize depression promotion, four theories have been offered: Neurotrophic, neurotransmitter, neuroendocrine, and anticonvulsant activity mechanisms. In neurotransmitter theory, it has been shown that the mechanisms of ECT are based on improving the production of neurotransmitters, including serotonin, adrenaline, and dopamine, or in the other way, by hypersensitivity of their receptors (6, 8). Also, it has been indicated that ECT can modulate excessive functional connectivity between key areas of the brain in depressed patients and affect cellular monitoring systems and intracellular regulation of brain-derived neurotrophic factor (BDNF) and neurotrophic factors (NTFs), which these factors could promote the neurogenesis and neuroplasticity in the prefrontal cortex area (6).
Despite the effectiveness of ECT in depressed patients, because of its retrograde forgetfulness side effect and patients’ fear due to ECT prescription, this method is less commonly used in despaired ones (9). Hence, investigators have tried to improve ECT’s positive points by diminishing its side effects.
Recent studies have illustrated that many plant compounds (fruits and vegetables), including polyphenols, flavonoids, essential oils, and alkaloids, could antagonize anxiety and depressant behaviors (1). It was shown that hesperetin as a flavonoid component could increase antioxidants such as superoxide dismutase (SOD), catalase, glutathione (GSH), and BDNF production, as well as decrease malondialdehyde (MDA) in the hippocampus and prefrontal cortex (10-12). Therefore, hesperetin can be a good candidate to improve positive effects and lessen ECT’s negative side effects.
2. Objectives
3. Methods
3.1. Administration of the Drugs
To induce depression in the animals, we used a chronic injection of reserpine (0.2 mg/kg; i.p, Sigma Aldrich, USA) every day for 14 days (13). In the treatment groups, hesperetin (10 or 20 mg/kg; Sigma Aldrich, USA) was dissolved in ethanol (5 mg/1 mL) and given to the animals once a day for a week (10, 12).
3.2. Experimental Animal Groups
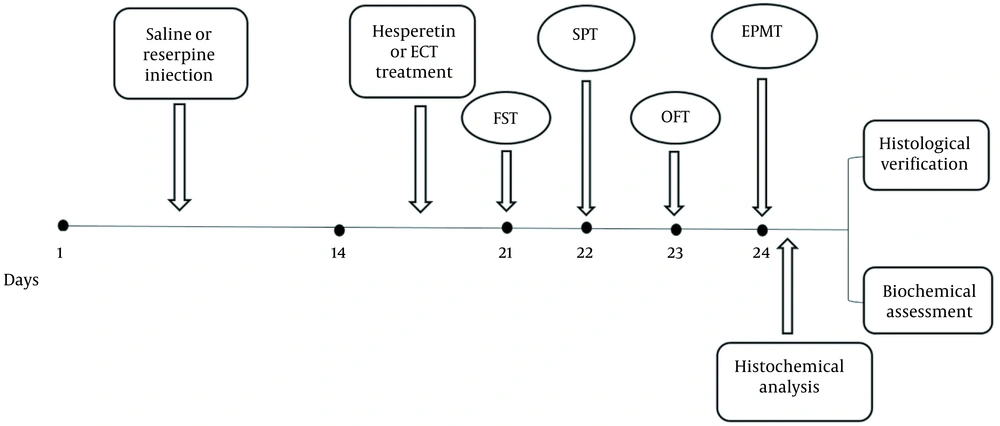
In this research, we only selected adult male rats (n = 80). The animals (250 - 230 g) were prepared from Karaj Razi Vaccine and Serum Research Institute (KRVSI) and kept for 7 days in a standard room at Shahed University Animal Laboratory to adapt before the tests. All experimental steps were approved by the Ethics Committee of Shahed University of Medical Sciences and performed according to the National Institute of Health’s Guide and Use of Laboratory Animals. Four animals were kept in each cage with access to enough water and food except during the behavioral test. The animals were divided into 8 groups (n = 10): (1) control (only received saline as the solvent of reserpine); (2) ECT; (3) reserpine (Res); (4) and (5) Res + hesperetin (Hes; 10 and 20 mg/kg; (6), Res + ECT; and (7) and (8) Res + ECT + Hes (10 and 20 mg/kg, respectively).
3.3. ECT Method
The electroshock was applied once a day for a week to the animals through ear clamp electrodes (15). The applied waves by the unit of electroshock instrument (Ugo Basile, Italy) were synced and quadrangular as follows: Amplitude 60 mA, width 0.5 ms, 100 Hz, and 1-second duration. The incidence of tonic-clonic convulsions with a period of fewer than 20 seconds without casualties showed the success of the method.
3.4. Behavioral Tests
All experimental groups underwent behavioral tests after 2 to 3 weeks. Figure 1 illustrates the timeline.
3.5. Forced Swimming Test
The forced swimming test (FST) is one of the most common and safest tests to evaluate rodent depression, which was performed within two days in this study. In this experiment, a plexiglass cylinder with a height of 60 cm and a diameter of 30 cm filled with water (25°C) near a 35 cm height of the cylinder was used. To acclimatize the animals to the watery environment, for 2 days, the rats were put in a cylinder filled with water for 15 minutes and then removed and placed at 30°C to dry. The time of immobilization of the rats was measured for 5 minutes. Normally, the immobility of the limbs and the floating of the animal in such a way that only the animal’s head came out of the water with very small movements were considered as immobility time (15).
3.6. Sucrose Preference Test
Anhedonia (inability to feel pleasure), which is a symptom of depression, was assessed by the sucrose preference test (SPT). Initially, we used 2 containers, one containing water and the other containing 1% sucrose. SPT was performed within 4 days. On the first day, 5 g of sucrose was dissolved in 500 mL of water in 2 separate containers to prepare a sucrose solution (1%. On the second day, one of the sucrose containers was replaced with an absolute water container to adapt the animals to the sucrose solution. On the third day, the animals were deprived of water and food. After this deprivation, on the fourth day, each rat had free access to 2 full bottles (one absolute water and the other sweet water) for an hour. Each bottle was weighed before and after the 1-hour water access period (16). Then, the sucrose preference percentage (SPP) was calculated according to the following equation:
3.7. Open Field Test
The open field box is a tool for measuring depression/anxiety behaviors. This box includes a square environment with a width of 72 × 72 and 36 cm high walls. The width of this device is divided into 16 squares (18 × 18 cm), with a square (18 × 18) in the center of the box. At the beginning of the experiment, the animal was gently placed in the center of the environment and freely searched the area for 5 minutes. Evaluation criteria in this test include the number of entrances to the center, the number of lines crossing the floor sections, and the duration of the time that an animal spends in the center. This experiment is based on the animal’s inherent fear of being in the center of the environment (11).
3.8. Elevated Plus Maze Test
The elevated plus maze (EPM) is made of 4 arms (2 open and 2 closed arms) crossing over each other. The open and closed arm dimensions were 10 × 50 cm, and there were 40 cm high walls on both sides and the ends of the enclosed arms. The 4 arms terminated in a central range of 10 cm. The maze was placed at the height of 50 cm above the floor using bases. Adequate light was provided by a 60-W light bulb that illuminates one side of the arm depending on the maze. For behavioral experiments, the animals were individually placed in the center of the maze plus facing the open arm, allowing them to search freely for 5 minutes. During this period, the number of entrances and the time spent in each arm was recorded in 5 minutes from a distance of 1 meter. The criterion for entering the arms was the rats’ hind paws passing from the entry of each arm. The duration of time spent in each arm was also calculated accordingly using a stopwatch. All measurements were performed in a very quiet atmosphere with consistent light. The open arm entries’ percentage (OAE%) for each animal was calculated according to the following equation (17).
3.9. Assessment of Oxidative Stress Biomarkers
Initially, the animals (5 rats in each group) were anesthetized by intraperitoneal injection of ketamine (10%) at a dose of 50 mg/kg. The animal was beheaded by a guillotine, and the brain was removed from the skull. The anterior part of the brain (+ 2.61 of bregma), which contains the mass of the prefrontal region, was carefully removed. The blocks were washed with a cold saline solution with radioimmunoprecipitation assay (RIPA) buffer (Sigma, Germany) and then homogenized with a homogenizer (IKA, Germany) at 5000 rpm for 2 minutes. Then, the homogenized tissue was mixed with phenylmethylsulfonyl fluoride (0.5 mM; Sigma Aldrich, Germany) as a protease inhibitor and centrifuge (1000 g, 10 min) at 4°C environment temperature. After centrifugation, the supernatant was separated from the solution by the sampler and kept at -70°C until testing. The lower pellet was discarded, and the supernatant was used to measure biochemical factors associated with oxidative stress, including SOD, MDA, GSH, and BDNF levels.
3.9.1. Malondialdehyde Assessment in the Prefrontal Cortex Area
Malondialdehyde (MDA) was estimated by measuring the thiobarbituric acid reactive substance (TBARS) method (7). Shortly after the addition of TBARS and trichloroacetic acid to the supernatant and uniform mixing of them, the solution was kept at 90°C for 80 minutes. Then the samples were cooled on ice and centrifuged by a freezer-centrifuge (4°C) at 1000 g for 10 minutes. Finally, the absorbance of solutes was read at 532 nm by a spectrophotometer (EX800, USA) instrument.
3.9.2. Measurement of Superoxide Dismutase Activity in the Prefrontal Cortex
A series of formulated brains’ supernatant samples were also used to measure the superoxide dismutase (SOD) activity at the prefrontal cortex. At first, the supernatants were incubated for 40 minutes with xanthine and xanthine oxidase in potassium phosphate buffer (pH = 7.8, 37 °C). Then, a yellow dye component [i.e., nitro blue tetrazolium (NBT)] was added to the complex solution. According to the percentage of SOD in the complex, the color of NBT is changed from yellow to dark blue, related to the level of formazan production in the solute. Finally, the absorbance of the solutes was measured at 550 nm using a spectrophotometer and reported as 1 nitrite unit of SOD activity (12).
3.9.3. Prefrontal Cortex Assessment of Glutathione
Prefrontal glutathione (GSH) levels were measured by the method described by Rotruck (12). Additional concentrations of the above substance were added to 5, 5’-dithio-bis- [2-nitrobenzoic acid], which reacted to form a yellow nitro-aqueous tetrazolium. Finally, the absorption was read at 412 nm, and the result was described as the concentration of GSH (12).
3.9.4. Prefrontal BDNF Level Measurement
After the preparation of the homogenized brain (which was accurately described in the previous section), the sample solution was centrifuged at 6000 rpm for 20 minutes, and, finally, its supernatant was collected and stored at -80°C (18). However, the brain BDNF level was measured using a BDNF ELISA kit (Sigma, USA, RAB 1138). In this regard, the supernatant samples were diluted to 1: 300, and then, according to the manufacturer’s protocol, the absorption of the solute was measured at a 450-nm wavelength using a microplate reader (BioTek, USA).
3.9.5. Histological Evaluation of the Prefrontal Cortex
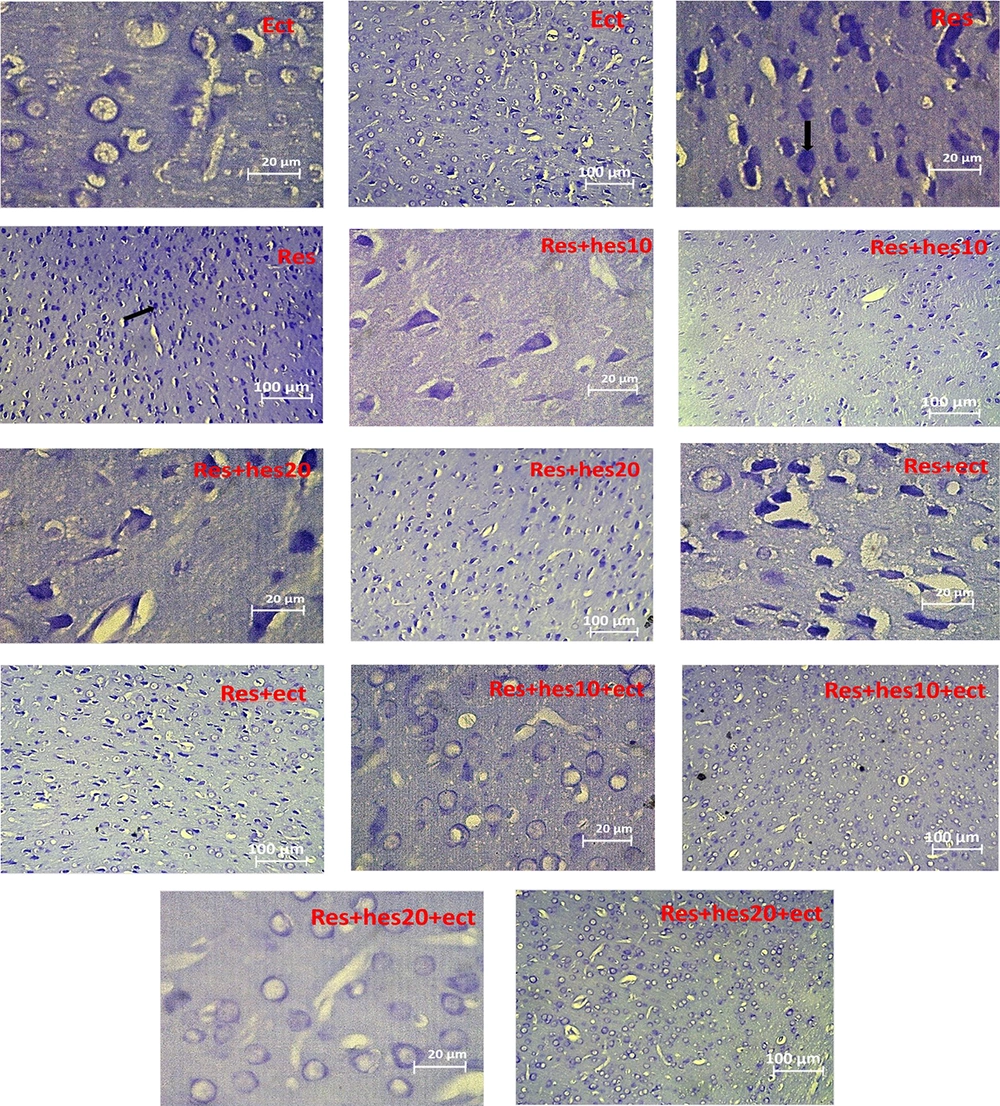
After completion of the behavioral experiments, the animal (n = 5) was severely anesthetized with ketamine (150 mg/kg), and due to an incision in the midline of the chest and pulling out the ribs, a heparin solution (0.1 mL) was injected from the apex of the heart in order to preclude thrombus. Then, the descending aorta was blocked by displacing the left lung, and the perfusion cannula was inserted into the left ventricle from the apex of the heart. Concurrently, during normal saline injection through the left ventricle to the upper section of the body, the right atrium was cut to drain the blood of the vessels and replace it with normal saline. Finally, after the complete extraction of the blood, the saline perfusion was stopped. Then, the animals were decapitated, and their brains were extracted and stored in 50 - 100 mL of 4% paraformaldehyde and phosphate buffer 0.1M solution (pH = 4.7). The brain tissue was drained with alcohol and then cleaned (dealcoholized) with xylene. Then, the anterior part of the prefrontal cortex (+ 2.61 from bregma) was removed and placed in impregnated paraffin at 56 - 60°C. After paraffin molding, the brain prefrontal sections (7-μm thickness) were cut using a rotating microtome (Leica, Germany) and placed on gelatin-coated slides; finally, the slides were subjected to Nissl staining. For this purpose, we stained the sections with cresyl violet 0.1% (Sigma Aldrich, USA), wrapped them with EntellanTM adhesive, and coated them with lamellae. The photographic study of the Nissl stained slides was examined under a microscope (magnification X40; AX70, OLYMPUS) using ImageJ software. The prefrontal cortex cell death was randomly counted in 6 - 7 slices with a minimum distance of 40 μ. In each slice, an area of 1250 μm2 was evaluated for counting the cell death. However, we consider the cell contraction, loss of Nissl body uniformity, cytoplasm and nucleus viscosity, and nucleus pyknosis as key parameters for introducing a cell as not being alive.
3.10. Statistical Analysis
Initially, the acquired data were analyzed for normality using SigmaStat version 3. The comparison between animal groups’ normal data was evaluated using a 2-way analysis of variance (ANOVA) and related post hoc tests. Also, nonparametric data were analyzed by Kruskal-Wallis and related tests. All data were presented as mean ± SEM, and P values less than 0.05 were considered statistically significant.
4. Results
4.1. FST Analysis
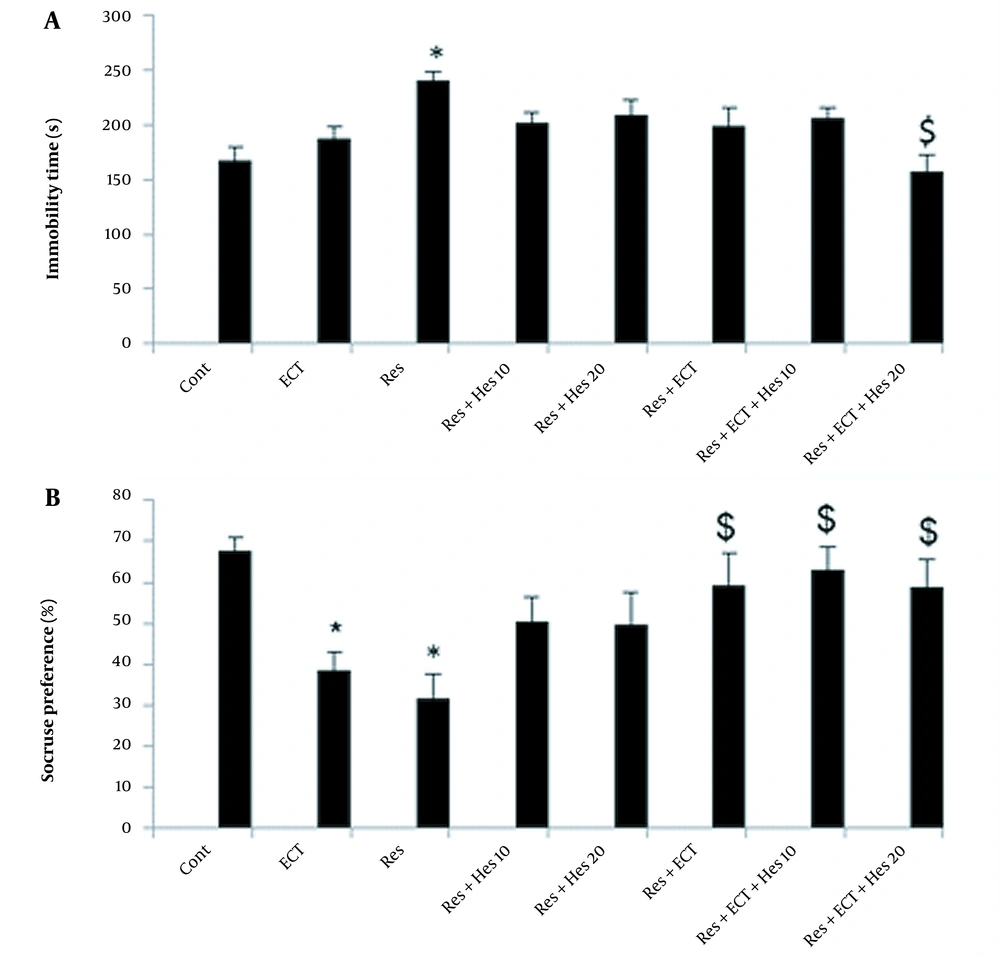
The immobilization time analysis report is shown in Figure 2A. As it is obvious, the reserpine could markedly increase this parameter (240 ± 8.72) compared to the control (167 ± 12.33) animals (P < 0.05). Nevertheless, this incremental effect of reserpine could be significantly alleviated in animals’ treatment by ECT + Hes (20 mg/kg) application (157 ± 14.60; P < 0.05).
A, Immobility time; and B, sucrose preference test data in the control and treatment groups (bars show data as mean ± SEM; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; * and $ P < 0.05, respectively, show differences in the control and reserpine groups; n = 10 in each animal group).
4.2. SPT Results
As shown in Figure 2B, in the reserpine and ECT groups, the sucrose consumptions were noticeably reduced to 31 ± 5.75 and 38.75 ± 4.05 compared to the control rats (67 ± 3.33; P < 0.05). Surprisingly, the tendencies to the over sucrose intake were observed in the Res + ECT and Res + ECT + Hes (10 and 20 mg/kg) animal groups near 59 ± 7.73, 63 ± 5.53, and 59 ± 6.53, respectively (P < 0.05).
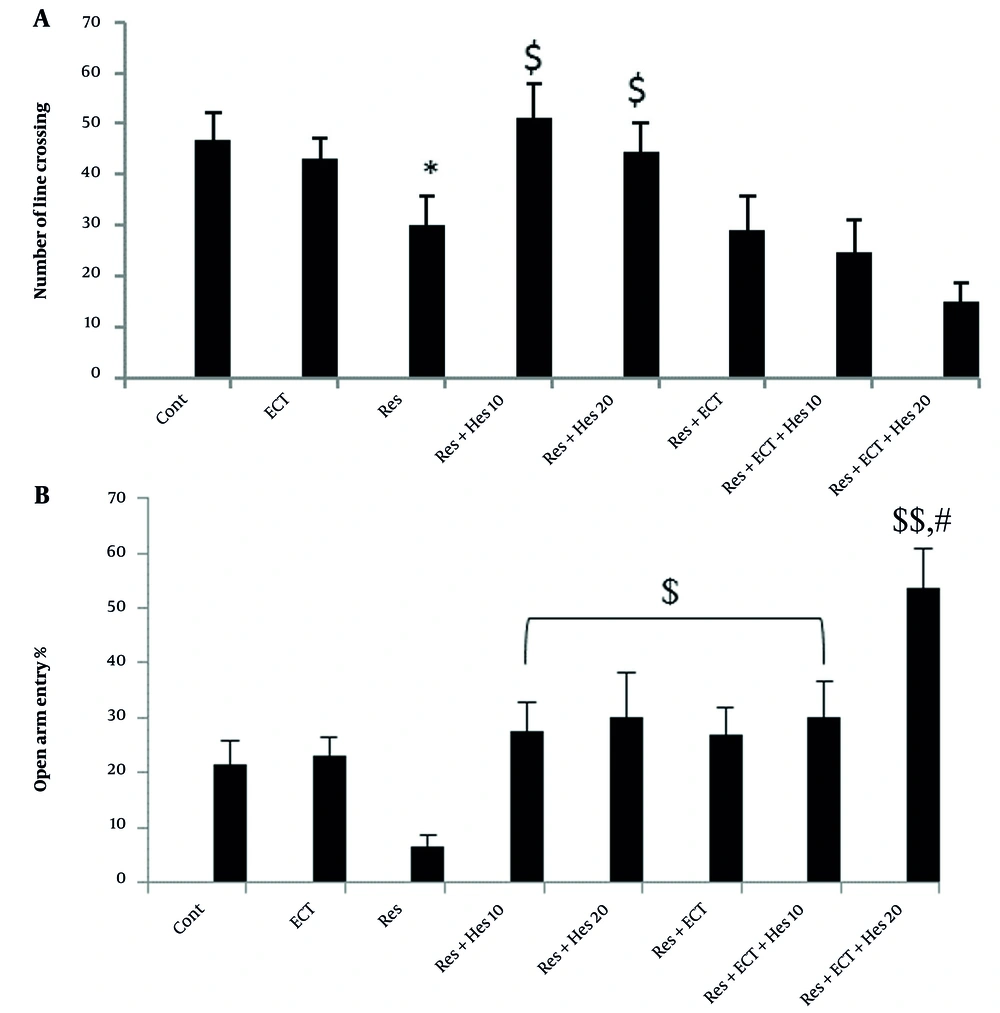
4.3. OFT
Statistical data indicated that the number of times crossing the lines of the floor in the device in animals treated with reserpine (30.2 ± 5.66) was reduced compared to the control group (46.7 ± 5.51; P < 0.05). The treatment of the depressed animals with hesperetin (10 and 20 mg/kg) notably increased the number of line crossings to 51 ± 6.72 and 44.55 ± 5.60 compared to the reserpine group (P < 0.05; Figure 3A).
A, The number of line crossings; and B, open arm entry percentage in open field and elevated plus maze tests (bars show the mean ± SEM data in each animal group; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; * P < 0.05, $ P < 0.05, and $$ P < 0.01 significantly indicated the difference between the mentioned bars and control and reserpine animals, respectively; # indicates a significance level vs Res + ECT, Hes (10 and 20 mg/kg), or ECT + Hes (10 mg/kg) groups; n = 10 in each animal group).
4.4. Obtained Data from EPMT
As indicated in Figure 3B, the OAE parameter significantly decreased to 6.64 ± 2.13 in depressed animals, but this reduction may be markedly diminished due to treatment by 10-mg/kg Hes, 20-mg/kg Hes, ECT, ECT + 10-mg/kg Hes, and especially more prominent by ECT + 20-mg/kg Hes near to 27.44 ± 5.40, 29.98 ± 8.18, 26.85 ± 5.03, 26.85 ± 5.03 and 53.52 ± 7.24, respectively (P < 0.05 and P < 0.01).
4.5. Oxidative Stress Biomarkers Data
Figure 4 shows the amounts of MDA, SOD, and GSH in the anterior prefrontal cortex of the experimental groups. As seen, MDA levels increased in the Res, ECT, Res + ECT, and Res + Hes (10 mg/kg) groups compared to the control animals (P < 0.05, 0.01, and 0.001). However, MDA levels could be reduced to normal values when the depressed animals were treated with Hes (10 and 20 mg/kg), ECT, and coadministration of hesperetin with ECT (P < 0.01, 0.001). Superoxide dismutase levels significantly decreased in the Res, Res + ECT, and Res + Hes (20 mg/kg) groups compared to the control ones. Also, there was a significant antagonizing activity for the decreasing effect of reserpine on SOD levels in the Hes (10 mg/kg) and ECT groups, as well as in the Hes (10 and 20 mg/kg), which were link applied with ECT (P < 0.01 and 0.001). We also found a marked reduction in GSH levels in depression animals, which was markedly antagonized by treatment in groups Hes (10 mg/kg) and Hes (20 mg/kg) + ECT (P < 0.05 and 0.001).
Malondialdehyde, superoxide dismutase, and glutathione stress oxidative biomarkers data in the anterior prefrontal cortex of the experimental groups [bars show the mean ± SEM of values; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; *, **, and *** P < 0.05, 0.01, and 0.001 (difference with the control); $, $$, and $$$ P < 0.05, 0.01, and 0.001 vs reserpine; ## (1, 2) and ### (1, 2) P < 0.01 and 0.001 vs Res + Hes (10 and 20 mg/kg) and finally, &&& P < 0.001 vs Res + ECT group; n = 5 in each group].
3.6. Prefrontal Assessment of Prefrontal Cortex BDNF
According to the biochemical results of the prefrontal cortex (Figure 5), the chronic use of reserpine significantly reduced BDNF levels (110.2 ± 9.20) compared to the control animals (145.7 ± 5.83; P < 0.05). In addition, BDNF decrement in the reserpine-treated rats was prominently prohibited in Hes (10 and 20 mg/kg) and ECT animal groups near 145.1 ± 3.80, 131.9 ± 4.18, and 142.1 ± 4.2, correspondingly. Accordingly, the measurement data for BDNF levels in Res + ECT + Hes (20 mg/kg) rats show no apparent changes, and it stands near to control ones (182.1 ± 4.39; P < 0.01).
Prefrontal brain-derived neurotrophic factor levels in the control and treatment animals [The bars indicate the mean ± SEM of data; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; * and *** P < 0.05 and 0.001 display the difference with the control. $, $$, $$$ show P < 0.05, 0.01, and 0.001 compared to the reserpine animals; ## P < 0.05 indicates a considerable difference compared to the Res + Hes (10 and 20 mg/kg), Res + ECT, and Res + ECT + Hes (10 mg/kg) groups; n = 5 in each group].
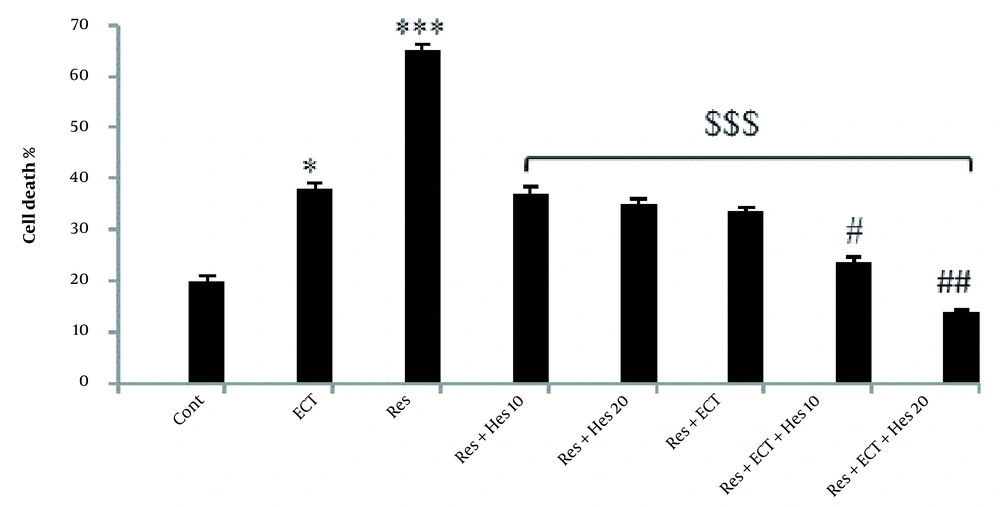
4.7. Neuronal Cell Death Assessment in the Prefrontal Cortex
As shown in Figures 6 and 7, the chronic application of reserpine or ECT alone could significantly enhance the number of cell death in the prefrontal cortex near 65.28 ± 1.08 and 37.94 ± 1.30, which are significantly more prominent than in the control rats (19.97 ± 0.98; P < 0.001). Thereby, the treatment of the animals with 10-mg/kg Hes, 20-mg/kg Hes, ECT, 10-mg/kg Hes + ECT, and 20-mg/kg Hes + ECT could antagonize reserpine-induced cell death (37.22 ± 1.10, 35.07 ± 1.10, 33.8 ± 0.8, 23.57 ± 1.10, and 13.88 ± 0.50, respectively; (P < 0.001).
The percentage of cell death in the prefrontal cortex in the control and treatment animals (Bars indicate the mean ± SEM of data; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; * and *** P < 0.05 and 0.001 compared to the control group; $$$ P < 0.001 with respect to the Res group; ## and ### P < 0.01 and 0.001 show the difference related to Res + Hes (10 and 20 mg/kg) and Res + ECT groups; n = 5 in each group).
Microscopic section of the prefrontal cortex, staining with the Nissl method for presentation of the number of cell death in the control and other treatment groups (the tip of the black arrow shows cell death numbers; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; n = 5 in each animal group).
5. Discussion
Consistent with previous studies, we showed that reserpine could induce depression response-linked behaviors like immobility time enrichment and reduction of freshwater cravings in rats (19-21). Also, in line with other studies, we showed an increase in prefrontal cortex cell death and anxiety-like behavior in reserpine-induced depressed rats (14, 21, 22). Also, consistent with other studies, we showed that a change in the production of oxidative stress biomarkers (MDA, SOD, and GSH) was associated with higher cell damage in the prefrontal cortex, which can finally lead to animal death (13, 23).
Overall, ECT is one of the most effective treatments for patients with treatment-resistant depression (TRD) conditions. ECT could regulate modulating changes in prefrontal cortex parameters, especially oxidative stress biomarkers in reserpine-treated animals (6). In our study, in the ECT animal group, a significant decrease in MDA levels and a prominent increase in SOD and BDNF in the prefrontal cortex in reserpine-treated animals were obtained (24). As a result, the higher digestion of free radicals ensures neuronal survival and, finally, can lower the rate of cell death in this area of the brain and enhance the response to ECT treatment. The mentioned results were mimicked by our obtained data from behavioral activity with respect to animals’ tendency to sucrose and an increase in motor activity in EPMT, which reiterated the worth of ECT for the treatment of depressed patients (5).
Overall, many reports show that the peel of citrus fruits, especially its main component (i.e., hesperetin), could yield a potent antioxidant activity; thus, we used this ingredient linked to ECT to improve the efficacy of ECT to treat TRD patients. In this setting, we found that the administration of hesperetin (10 and 20 mg/kg) could significantly reduce MDA levels and increase SOD, GSH, and BDNF in the prefrontal cortex. Also, our data on histological studies indicated a significant reduction in cell death in the prefrontal cortex in hesperetin-treated animal groups (11, 12). It was reported that this effect might be controlled by oxidative stress, suppressing activity or neuro-inflammatory modulating activity of hesperetin by regulation of NrF2/Ho-1 and TLR4/NF-κB signaling pathways and eventually by the prohibition of pro-inflammatory cytokines (TNF-α and IL-1β) (10, 25). In the depressed animals, we observed anxiety-like behaviors as a side effect of depression which could be suppressed by hesperetin. Meanwhile, according to depression-induced methods, anxiety may be occurred or not. As a result, according to the literature, we can conclude that the therapeutic results may differ depending on the type of depression, treatment protocols, profiles of treatments, and kinds of animals, which leads to a different activation of signaling pathways (11, 26-28). In this study, hesperetin administration in ECT-treated animals could significantly increase BDNF levels compared to other treatment groups, which may be a reason for the potent neurogenesis and synaptic plasticity in the prefrontal cortex in the ECT + Hes group. In addition, this group showed a significant increase in SOD and GSH and a marked decrease in MDA parameters according to the biochemical findings. Thus, it is thought that the suppression of depression-linked behavior as observed in immobility time, animal’s tendency to freshwater and motor activity tests could be wise and related to neuroprotection, reduction of inflammatory cytokines, and NF-κB expression in CNS, especially in the prefrontal cortex (10, 25, 29). As a result, the simultaneous use of ECT and hesperetin protocol in depressed animals can prevent the effect of free radicals on cellular macromolecules by strengthening the antioxidant system and neurotrophic growth factors and possibly reducing pro-inflammatory factors; ultimately, this protocol leads to a reduction of MDA and cell death in the forebrain area and improvement of depressive responses and anxiety-like behavior in animals treated with reserpine.
5.1. Conclusions
Consistent with previous studies on the efficacy of ECT and chronic administration of hesperetin in the treatment of depression, this study indicated that combination treatment of depression by ECT and chronic administration of hesperetin could significantly antagonize depression and anxiety-linked behaviors through the improvement of the prefrontal cortex antioxidant, BDNF production and hence prohibition of cell death in this region.
![Malondialdehyde, superoxide dismutase, and glutathione stress oxidative biomarkers data in the anterior prefrontal cortex of the experimental groups [bars show the mean ± SEM of values; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; *, **, and *** P < 0.05, 0.01, and 0.001 (difference with the control); $, $$, and $$$ P < 0.05, 0.01, and 0.001 vs reserpine; ## (1, 2) and ### (1, 2) P < 0.01 and 0.001 vs Res + Hes (10 and 20 mg/kg) and finally, &&& P < 0.001 vs Res + ECT group; n = 5 in each group]. Malondialdehyde, superoxide dismutase, and glutathione stress oxidative biomarkers data in the anterior prefrontal cortex of the experimental groups [bars show the mean ± SEM of values; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; *, **, and *** P < 0.05, 0.01, and 0.001 (difference with the control); $, $$, and $$$ P < 0.05, 0.01, and 0.001 vs reserpine; ## (1, 2) and ### (1, 2) P < 0.01 and 0.001 vs Res + Hes (10 and 20 mg/kg) and finally, &&& P < 0.001 vs Res + ECT group; n = 5 in each group].](https://services.brieflands.com/cdn/serve/3170b/8239bc772eb4a2c99ab76837398fbf0ae4b3d9eb/ijpbs-122915-i004-F4-preview.webp)
![Prefrontal brain-derived neurotrophic factor levels in the control and treatment animals [The bars indicate the mean ± SEM of data; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; * and *** P < 0.05 and 0.001 display the difference with the control. $, $$, $$$ show P < 0.05, 0.01, and 0.001 compared to the reserpine animals; ## P < 0.05 indicates a considerable difference compared to the Res + Hes (10 and 20 mg/kg), Res + ECT, and Res + ECT + Hes (10 mg/kg) groups; n = 5 in each group]. Prefrontal brain-derived neurotrophic factor levels in the control and treatment animals [The bars indicate the mean ± SEM of data; Abbreviations: Cont, control; ECT, electroconvulsive therapy; Hes, hesperetin; Res, reserpine; * and *** P < 0.05 and 0.001 display the difference with the control. $, $$, $$$ show P < 0.05, 0.01, and 0.001 compared to the reserpine animals; ## P < 0.05 indicates a considerable difference compared to the Res + Hes (10 and 20 mg/kg), Res + ECT, and Res + ECT + Hes (10 mg/kg) groups; n = 5 in each group].](https://services.brieflands.com/cdn/serve/3170b/5756fde5c79294d893466994e2dd09894035dc00/ijpbs-122915-i005-F5-preview.webp)