1. Background
Toxoplasma gondii is a zoonotic intracellular protozoan parasite with medical and veterinary importance worldwide that infects humans and many warm-blooded animals as the intermediate hosts and Felidae family members as the final host (1-3). The main routes of transmission to humans and other hosts are the ingestion of infective oocysts excreted in cat feces, consumption of tissue cysts in raw or undercooked meat, and congenitally (4, 5). Due to serious and life-threatening complications, toxoplasmosis is very important in people with defective immune systems, patients infected with highly virulent strains, and congenitally-infected fetuses and newborns (6, 7), while it is usually asymptomatic and self-limiting in healthy people (8). Toxoplasma gondii has three major clonal lineages (Types I, II, and III) related to mouse virulence. Totally, Type I strains are considered high virulent, whereas Types II and III strains are regarded as low to mild virulent in mice (9, 10).
Alzheimer's disease (AD) is one of the common age-related chronic progressive neurodegenerative diseases characterized by permanent reductions in cognitive abilities, such as attention, decision making, learning, and memory with unknown multifactorial etiology (11, 12). There are many ambiguities about the pathogenetic mechanisms of the disease (13, 14). What is widely agreed is that amyloid-beta (Aβ) oligomers resulting from the cleavage of the amyloid precursor protein (APP) by β- and γ-secretase enzymes accumulate as plaques, which are the main initiators of the pathological process of the disease and play a major role in neuronal damage and degeneration (15, 16). Neuronal degeneration is caused by neuroinflammation playing an important role in the pathogenesis of chronic neurodegenerative diseases, such as AD (17, 18). Recruitment of peripheral immune cells to the central nervous system increases the number of active microglia and their accumulation around Aβ plaques to clear them which is not very successful. These changes increase the production of inflammatory mediators, including cytokines, chemokines, complement factors, radical oxygen species, and NO (18-21). Most of these mediators are toxic to nerve cells and also stimulate amyloid precursor protein processing, thereby intensifying the process of neuronal degeneration and worsening the disease (17-21).
Toxoplasma gondii is a neurotropic parasite with lifelong persistence in the host brain that can affect brain function (22-25). Parasitic cysts can alter gene expression and affect various biological functions of neurons, such as the synthesis of neurotransmitters, neuronal circuitry, and synaptic plasticity, leading to the disruption of brain connectivity and behavioral deficits (26-31). The infection causes behavioral and cognitive dysfunction (29, 31-35) by manipulating the activity of essential molecules and pathways of the host body, such as altering acetylcholinesterase activity (36). This enzyme hydrolyzes acetylcholine, the main role of which is to regulate behavioral and cognitive functions (36).
In addition to the defense role against the parasite, host immune responses are another important factor that can damage self-cells and -tissues and alter their function (25, 28, 29). However, what is interesting is how the immune responses and other changes induced by T. gondii can affect the course of AD if both diseases exist simultaneously. Several studies have examined this issue in humans and animal models (37-42). Research on animal models has shown that toxoplasmosis plays a protective role in AD and reduces the neurodegeneration process by reducing the accumulation of plaques (37, 38). Some authors have argued that increasing anti-inflammatory cytokines (TGF-β and IL-10) and subsequently immunosuppression induced by the chronic form of the parasite in the brain reduces neuroinflammation and AD symptoms (37). The other study identified the successful clearance of plaques by the phagocytic system as the reason for the reduced pathogenesis of AD (38). Cabral et al. showed that the protective effect of toxoplasmosis against AD was specific to Type II strains and could not be due to an increase in anti-inflammatory cytokines (39). Another study showed that chronic T. gondii infection has a detrimental role and induces cognitive impairments in the brain of mice by causing neuroinflammation through inflammatory cytokines (40).
2. Objectives
Due to limited studies and disagreements in their findings, as well as the presence of many ambiguities, it seems necessary to conduct more comprehensive studies to clarify the ambiguities and achieve reliable and repeatable findings. Since different strains of T. gondii are classified according to their degree of pathogenicity and trigger different immune responses in the host body, the chronic infection caused by them may also play a different role in AD pathogenesis. Therefore, the present study aimed to investigate the effects of chronic toxoplasmosis infection with Types I (RH), II (PRU), and III (VEG) strains alone and in combination on cognitive impairments in the Alzheimer's rat model.
3. Methods
3.1. Animals
The current study was conducted according to institutional animal ethics guidelines of the Animal Research Center, Mazandaran University of Medical Sciences, Sari, Iran (ARCMUMS). The experimental protocols for animal use in this study were approved by the Mazandaran University of Medical Sciences Ethics Committee (MUMSEC), Sari, Iran (IR.MAZUMS.REC.1399.608).
A total of 70 adult male Wistar rats at the age of 8 - 10 weeks and weight of 200 ± 20 g were obtained from the Institute of Laboratory Animals of Mazandaran University of Medical Sciences, Sari, Iran. The animals were kept at the Institute under controlled conditions (23 ± 2°C, humidity 45 ± 5%, and 12 hr Light/dark cycle) with access to food and water ad libitum.
3.2. Experimental Design
Figure 1 outlines the experimental timeline. The rats were randomly divided into 10 groups of 7 each (n = 7) as follows: (1) sham group: Rats without parasite inoculation that underwent sham surgery (without intrahippocampal [IH] injection), (2) vehicle group: Rats without parasite inoculation and with IH injection of Phosphate-Buffered Saline (PBS), (3) Aβ group: Rats without parasite inoculation and with amyloid beta 1-42 (Aβ1-42)-induced AD, and (4 - 10) AβRH, AβPRU, AβVEG, AβRH + PRU, AβRH + VEG, AβPRU + VEG, AβRH + PRU + VEG groups: Rats inoculated with RH, PRU, and VEG strains of T. gondii alone or in combination and with Aβ1-42-induced AD.
3.3. Chronic Models of Toxoplasma gondii Infection
RH, PRU, and VEG strains of T. gondii were used to induce chronic toxoplasmosis infection in rats. The strains were provided by Professor Marie-Laure Darde (Head of Biological Resource Center for Toxoplasma, Limoges University, France).
To prepare parasite cysts, the brains of mice infected with each of PRU and VEG strains were separately homogenized in 1 mL normal saline and counted under a light microscope with a 10 × objective lens, then, 20 - 25 cysts were injected intraperitoneally into adult mice. Three months after injection, the brains of infected mice were removed and after preparation, their cysts were counted and used for injection into rats. The number of cysts required from each PRU and/or VEG strain for injection into experimental groups was as follows: 100 cysts into AβPRU and AβVEG groups; 50 cysts into AβRH + PRU, AβRH + VEG, and AβPRU + VEG groups; and 33 cysts into AβRH + PRU + VEG group.
To provide RH strain tachyzoites, about 3 - 5 days after intraperitoneal injection of 1 × 106 tachyzoites in sterile PBS (pH = 7.4) containing 100 IU/mL penicillin and 100 mg/ml streptomycin, the peritoneal fluid of infected mice was drawn with a syringe and counted under a light microscope with 40 × objective lens and got ready to be injected into rats. Finally, 106, 5 × 105, and 3.3 × 105 tachyzoites of RH strain were injected into experimental groups that were to be infected with one (AβRH group), two (AβRH + PRU and AβRH + VEG groups), and three strains (AβRH + PRU + VEG group), respectively.
3.4. Animal Model of AD
Alzheimer's induction in rats was performed by injecting human Aβ1-42 peptide into the brain hippocampus after the development of chronic toxoplasmosis (7 months after parasite inoculation).
In the first step, human Aβ1-42 (1 mg; BioLegend, San Diego, CA, USA) was dissolved in 1 mL of 1% NH4OH and PBS according to the manufacturer's instructions. The final concentration of Aβ1-42 was 1 μg/μL, and it was incubated at 37°C for 7 days to aggregate into soluble neurotoxic oligomers. The rats were anesthetized by the injection of ketamine (60 mg/kg) and xylazine (10 mg/kg) intraperitoneally. The animal head was fixed in a stereotaxic apparatus and Aβ1-42 (5 μL/hemisphere) was injected bilaterally into the hippocampus CA1 using a Hamilton syringe according to Paxinos and Watson (AP = 3.9 mm, LR = 2.2 mm, and D = 2.7 mm) (43). The injection rate was 0.5 μL/min, and the needle was left in place for 3 - 5 min to avoid reflux before being slowly retracted.
3.5. Behavioral Tests
Ten days after stereotaxic surgery, animals were subjected to the elevated plus maze (EPM) and Morris water maze (MWM) to determine anxiety-like behaviors, and spatial learning and memory, respectively.
3.5.1. Elevated Plus Maze
The EPM test is used to assess anxiety-like behavior in the rodent models of central nervous system disorders. EPM apparatus was made of black wood consisting of a central platform (10 × 10 cm) associated with two open arms (50 × 10 cm) and two closed arms (50 × 10 × 50 cm) with a height of 50 cm above the floor. Rats were individually placed in the central platform between the open and closed arms facing an open arm and allowed to explore the maze for 5 min. By analyzing the video recorded by the camera installed on the top of the maze, the time spent, as well as the number of entries in the open and closed arms were recorded. It was defined as arm entry when a rat placed its all four paws into an arm. After each test, the apparatus was cleaned with a 70% ethanol solution and dried (44).
3.5.2. Morris Water Maze
Morris Water Maze task was used to assay spatial learning and memory, as well as its classic version (within 5 successive days) was done. The maze consisted of a circular tank (150 cm in diameter and 50 cm in height) filled with water (25 ± 2°C) to a level of 20 cm from the edge of the tank. The tank was divided into four quadrants, one of which contained a transparent circular Plexiglas platform (15 cm diameter) placed at a fixed position, 2 cm below the surface of the water in the southwest quadrant of the tank (target quadrant). Visual cues were fixed at different walls surrounding the tank.
On the first day (adaptation phase), the animals were allowed to swim in the water for 60 sec without a platform in order to adapt. For the next three days (spatial learning phase), four trials (one block) were performed each day (totally three blocks in three days) and in each trial, which lasted 60 sec, the animal was released from one of the quadrants into the pool. If the rat was able to find the platform within the given time, it was allowed to stay on the platform for 20 - 30 sec. The interval between trials was 30 sec. The parameters of swimming speed, time spent, and the traveled distance (path length) to find the platform were recorded using a camera mounted on the top of the maze and a video tracking system (Borj Sanat, Iran). On the last day (long-term spatial memory phase, probe test), the platform was removed from the target quadrant, and the rats were released from the quadrant in front of the target quadrant into the water, allowing them to search for the platform for 60 sec. Parameters recorded for this step were time spent, the traveled distance, and the number of crossing (Frequency) in the target zone (45). At the end of the probe trial, a visible platform test was conducted to reveal any possibility of intervention with sensory and motor coordination or motivation. In this test, the platform was raised 2 cm above the water surface in the quadrant located opposite the prior position, and the ability of rats to escape to a visible platform was determined (46).
3.6. Statistical Analysis
The obtained data were analyzed in SPSS software (version 26.0) through one- and two-way analysis of variance (ANOVA) and repeated measures. Analyses of variances were followed by Tukey's post hoc test for multiple comparisons. Data were expressed as a mean ± SD, and a p-value less than 0.05 was considered statistically significant.
4. Results
4.1. Anxiety-like Behaviors
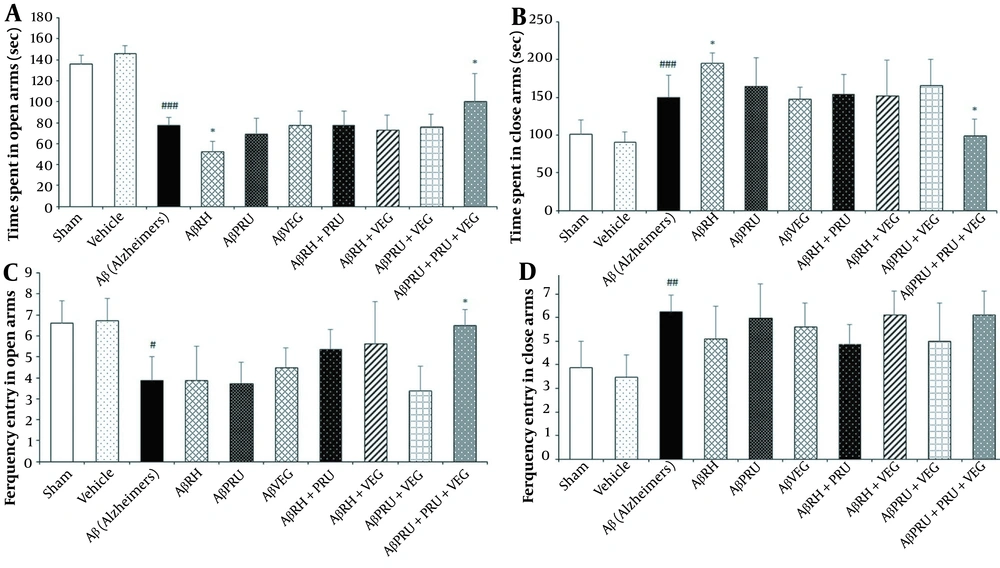
The EPM test was conducted to assess anxiety-like behavior and the data related to this test was analyzed with one-way ANOVA. In the test, the Aβ group exhibited more anxiety-like behavior than the vehicle and sham groups in all of the EPM parameters tested (Figure 2). The AβRH group showed increased anxiety-like behavior with a significant decrease in time spent in open arms compared to the Aβ group (P < 0.05, Figure 2), which indicates that infection with RH strain has exacerbated the anxious behavior of rats with AD. In addition, an increase in time spent in open arms was observed in the AβRH + PRU + VEG group, compared to the Aβ group (P < 0.05, Figure 2), demonstrating decreased anxiety-like behavior in rats infected with all three strains of T. gondii. The anxiety-like behavior in the other groups showed no difference, compared to the Aβ group.
The effects of chronic toxoplasmosis infection with RH, PRU and VEG strains alone and in combination on anxiety-like behavior of Alzheimer's rat model in the Elevated plus maze: (A) time spent in open arms, (B) time spent in close arms, (C) ferquency entry in open arms and (D) ferquency entry in close arms. Data represent the mean ± SD. * P < 0.05, in comparison with the Alzheimer's group. # P < 0.05, ## P < 0.01, ### P < 0.001, in comparison with vehicle group.
4.2. Spatial Learning
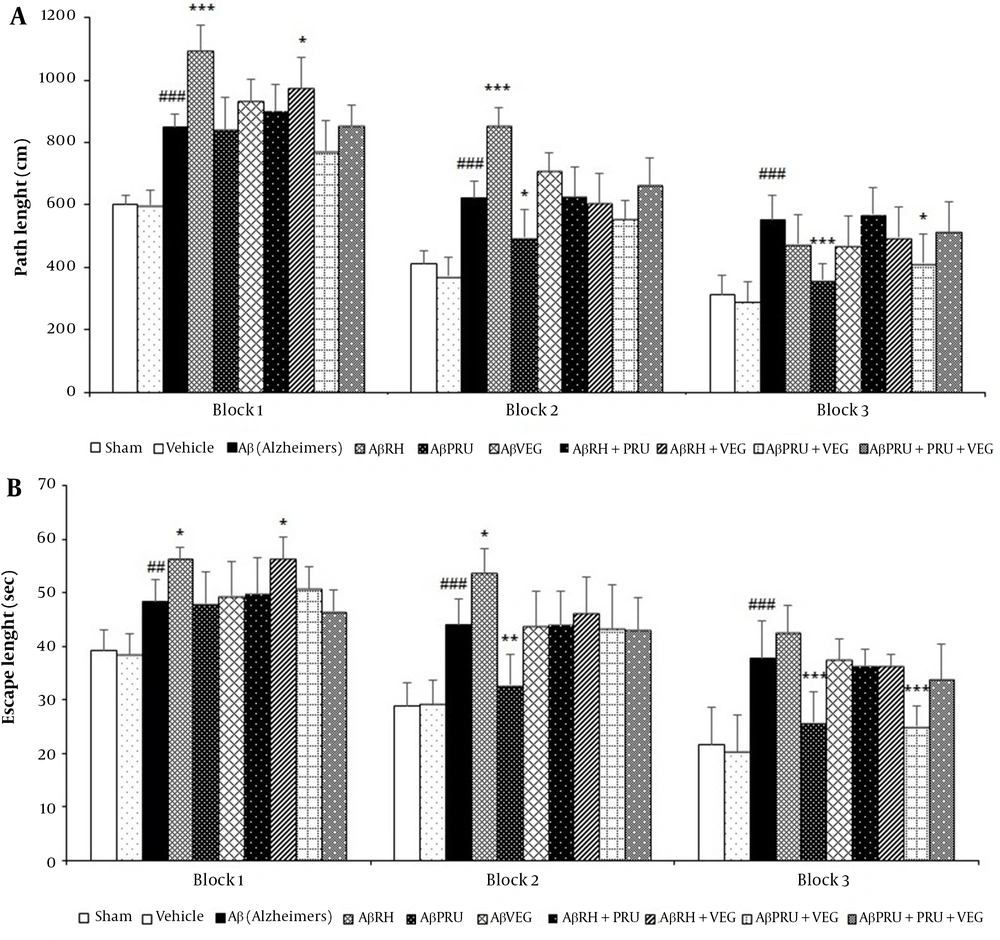
Statistical analysis of two-way ANOVA confirmed that rats in the Aβ group had higher mean path length and escape latency to find the hidden platform in the MWM, compared to the vehicle and sham groups, while the control and sham groups were not different in this regard (Figure 3).
The effects of chronic toxoplasmosis infection with RH, PRU and VEG strains alone and in combination on spatial learning of Alzheimer's rat model in the Morris water maze: (A) path length and (B) escape latency. Data represent the mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001, in comparison with the Alzheimer's group. # P < 0.05, ## P < 0.01, ### P < 0.001, in comparison with vehicle group.
The path length and escape latency of the AβRH group significantly increased in blocks 1 (P < 0.001 and P < 0.05, respectively) and 2 (P < 0.001 and P < 0.05, respectively) in comparison with the Aβ group, which indicates spatial learning impairments and intensification of the AD process following toxoplasmosis infection with RH strain (Figure 3). However, the analysis showed a protective role of PRU strain in AD because the path length and escape delay of the AβPRU group significantly decreased in blocks 2 (P < 0.05 and P < 0.01, respectively) and 3 (P < 0.001 and P < 0.001, respectively), compared to the Aβ group (Figure 3). Although rats infected with VEG strain (AβVEG group) showed an increase in the distance traveled to reach the platform in blocks 1 and 2, compared to the Aβ group, this difference was not statistically significant (Figure 3). Among the groups infected with the strains of T. gondii in combination, the AβRH + VEG group significantly reduced spatial learning only in block 1, and the AβPRU + VEG group showed increased spatial learning only in block 3. The low differences were also observed in the two groups of AβRH + PRU and AβRH + PRU + VEG, compared to the Aβ group that were not statistically significant (Figure 3). It should be noted that the swimming speed was the same for all groups in all trials.
4.3. Spatial Long-Term Memory
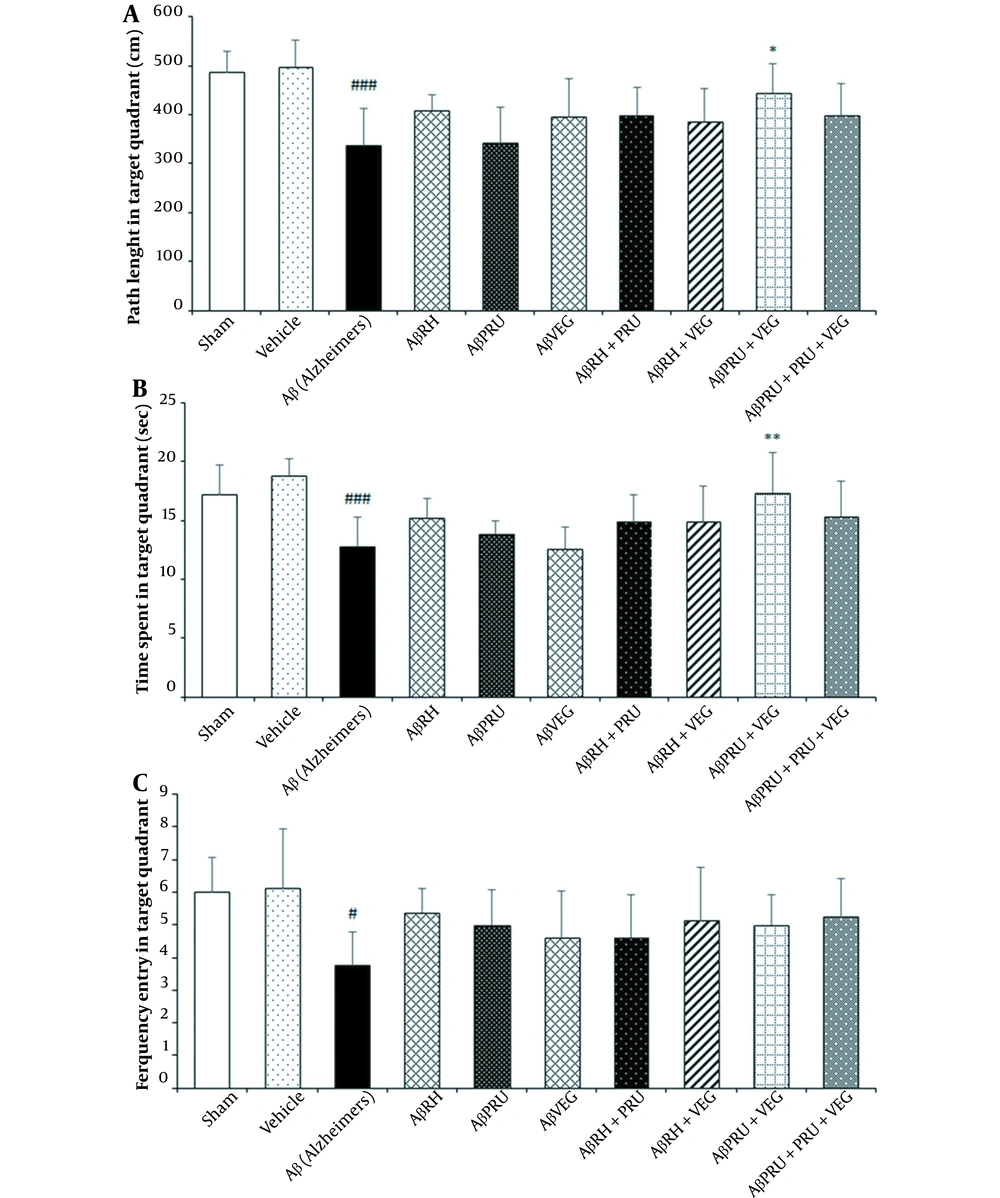
One day after the learning phase, a probe test was performed to assess the long-term memory of rats. The time and distance spent and the number of crossing in the target quadrant (frequency) were evaluated for spatial long-term memory retention.
Using one-way ANOVA analysis, as expected, the difference of the Aβ group with the vehicle and sham groups was significant for all three parameters, while the difference between the vehicle and sham groups was not significant (Figure 4). The rats in each group presented similar time, distance spent, and frequency of entry in the target quadrant in the probe test. With the retention of long-term memory, the AβPRU + VEG group reduced the memory impairments caused by AD; moreover, it had been able to improve learning since the rats of this group spent more time (P < 0.01) and distance (P < 0.05) in the target quadrant in comparison with the Aβ group (Figure 4). Although the other groups had differences from the Aβ group on the parameters of the probe test, the differences were not statistically significant (Figure 4).
The effects of chronic toxoplasmosis infection with RH, PRU and VEG strains alone and in combination on spatial memory of Alzheimer's rat model in the Morris water maze: (A) path length, (B) escape latency and (C) frequency entry in target quadrant. Data represent the mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001, in comparison with the Alzheimer's group. # P < 0.05, ## P < 0.01, ### P < 0.001, in comparison with vehicle Group.
4.4. Latency to the Visible Platform and Swimming Speed
The one-way ANOVA analysis of escape latency to reach the visible platform (P > 0.05) and swimming speed (P > 0.05) revealed no significant difference among groups (Table 1), representing T. gondii inoculation and AD induction had no effects on visual and motor functions, and all groups were similar in this respect.
| Groups | Swimming Speed (cm/s) b | Escape Latency (s) b |
|---|---|---|
| Sham | 22.20 ± 2.8 | 19.66 ± 3.32 |
| Vehicle | 22.85 ± 2.6 | 19.74 ± 2.38 |
| Aβ (Alzheimer's) | 20.11 ± 2.4 | 20.56 ± 2.33 |
| AβRH | 23.13 ± 1.8 | 19.94 ± 3.07 |
| AβPRU | 21.04 ± 2.05 | 21.16 ± 1.39 |
| AβVEG | 20.15 ± 3.3 | 20.2 ± 1.82 |
| AβRH + PRU | 21.46 ± 1.8 | 21.5 ± 1.16 |
| AβRH + VEG | 20.86 ± 2.6 | 19.9 ± 2.17 |
| AβPRU + VEG | 19.68 ± 1.9 | 21.5 ± 1.35 |
| AβRH + PRU + VEG | 20.89 ± 2.3 | 20.5 ± 1.3 |
aValues are expressed as mean ± SD.
b The P-value for all groups compared to the vehicle group was greater than 0.05.
5. Discussion
Toxoplasma gondii is a neurotropic parasite with lifelong persistence in the host brain that can affect brain function. Chronic infection causes behavioral and cognitive dysfunction by manipulating the activity of essential molecules and pathways of the host body and subsequently nerve degeneration (29, 31-35). On the other hand, Toxoplasma often infects the areas of the brain that are affected by AD, such as the hippocampus (47). Many researchers suggested toxoplasmosis as a risk factor for the development of neurodegenerative and psychiatric disorders, such as AD; however, the link between toxoplasmosis and AD has not been fully elucidated (26, 48). Therefore, the present study was designed to investigate the effects of chronic toxoplasmosis infection with Types I (RH), II (PRU), and III (VEG) strains alone and in combination on cognitive impairments in Alzheimer's rat model.
Our findings showed that chronic toxoplasmosis infection with RH strain increased anxiety-like behavior in Alzheimer's rats in the EPM. In addition, a decrease in anxiety-like behavior was observed in the group infected with RH, PRU, and VEG strains in combination. In agreement with the EPM findings, infection with the RH strain led to the worsening of spatial learning impairments in the Alzheimer's rat model in the MWM task; however, it did not affect spatial memory as demonstrated in the probe test. Conversely, infection with the PRU strain significantly enhanced spatial learning in the test without being able to improve memory impairments. Improvement in spatial learning and memory impairments was also observed in Alzheimer's rats infected with PRU and VEG strains in combination. Infection with RH and VEG strains in combination relatively exacerbated the spatial learning impairments caused by AD that was less than the effect of RH strain. On the other hand, chronic infection with VEG strain did not show a significant effect on cognitive disorders of the Aβ1-42-induced AD model rats.
Numerous studies have focused on the association between toxoplasmosis and AD in humans and animal models; however, their findings have been controversial (37-42). In agreement with our findings, research on animal models has shown that toxoplasmosis with the ME49 Type II strain plays a protective role in AD and reduces the neurodegeneration process by reducing the accumulation of plaques (37, 38). One of these studies showed attenuated spatial learning and memory impairments in Tg2576 mice infected with T. gondii by the water- and Y-maze tests (37). The authors of this study have argued that increasing anti-inflammatory cytokines (TGF-β and IL-10) and subsequently immunosuppression induced by the chronic form of the parasite in the brain reduces neuroinflammation and AD symptoms (37). The other study identified the successful clearance of plaques by the phagocytic system as the reason for the reduced pathogenesis of AD (38). Cabral et al. showed that the protective effect of toxoplasmosis against AD was specific to Type II strains and could not be due to an increase in anti-inflammatory cytokines (39). Conversely, Mahmoudvand et al. illustrated that chronic T. gondii infection had a detrimental role and induced cognitive impairments in the brain of mice by causing neuroinflammation through inflammatory cytokines in the MWM test (40).
As noted, our findings of the protective role of the Type II strain of T. gondii in AD pathogenesis are consistent with the results of most studies (37-39); however, there have been studies with different results (40). Various reasons for these differences can be defined, the most important of which are the type of animal used, the dose and strain of T. gondii, type of induction of Alzheimer's model, stage of AD pathogenesis, and chronic toxoplasmosis period during experiments (49, 50).
In general, the protective role of type II strains in the studies can be due to the establishment of an immune environment in the brain that is in favor of AD and ultimately reduces neuronal degeneration caused by the deposition of amyloid plaques. On the other hand, our findings on the detrimental role of Type I (RH strain) of T. gondii against AD can be considered very important because it has not been studied so far. Since diverse strains of T. gondii have distinct pathogenicity degrees and trigger different immune responses in the host body, it can be said that the immune responses and other interactions triggered by RH strain are distinct from the changes induced by PRU strain that lead to increased impairments and exacerbation of the neurodegenerative process of AD.
Furthermore, the sum of the interactions created in the host body by chronic infection with a combination of the strains determines their protective or destructive role in AD, and relating the overall effect to a particular strain is difficult and complex since the effect of one strain when it causes a chronic infection alone may be very different from that when it creates together with the others. The total of direct and indirect changes (e.g., alterations in the expression of many genes, the production of cytokines, neurotransmitters, enzymes, etc.) (26-31, 36, 37) resulting from infection with one strain alone ultimately lead to an overall outcome on AD pathogenesis that may exacerbate or improve the disease or have no effect on it. But when two or more strains infect the host, changes made by them together determine the course of AD, which may be different in type and amount from effects of each strain alone. For example, the findings of our study showed increased anxiety-like behavior in rats infected with RH strain in the EPM, whereas when RH strain was combined with PRU and VEG strains, the anxiety-like behavior of animals decreased.
5.1. Conclusions
In conclusion, the present findings demonstrated that chronic infection with the PRU strain of T. gondii protects against cognitive impairments of AD, while RH strain plays a detrimental role in AD pathogenesis. In addition, infection with PRU and VEG strains in combination significantly improves spatial learning and memory deficits in Alzheimer's rats. Relative detrimental and protective effects on AD pathogenesis were also observed for the groups of AβRH + VEG and AβRH + PRU + VEG, respectively. The VEG strain had no significant effect on AD pathogenesis. Further studies are required to determine which types of mechanisms are involved in the effects of these strains against Aβ1-42-induced cognitive impairments.