1. Background
Substance use disorder is one of the most critical problems spreading worldwide and plaguing nations. Among drug users, stimulants, especially methamphetamine (METH), a widely used worldwide (1, 2). According to Iranian research, METH is the country's third most frequently used drug, making up approximately 3.6% of the total volume of used substances (3).
The abuse and dependence on substances, especially METH, has posed a serious challenge to researchers and those involved in mental health (4). METH can increase wakefulness, reduce appetite, interfere with the heartbeat, raise blood pressure, and trigger hyperthermia (increased body temperature). People using such substances for a long time may experience severe weight loss, major dental problems, insomnia, confusion, and violent behavior (5).
One of the most pressing and consistent topics during the treatment of addictive disorders is craving, temptation, or the desire to resume consuming. Losing addictive behaviors (such as substance and alcohol use disorder and gambling) seems relatively common among drug addicts (6). Different approaches are applied to evaluate multiple aspects of substance use disorder. Considering that the influential factors in drug use initiation, continuation, and relapse are very diverse and include various individual, family, and social factors in treating drug users apart from psychological and social aspects, the pharmacological factors should also be considered (7).
Several effective medications have been studied for drug use treatment (8). However, researchers are looking for an alternative medication (such as methadone in quitting morphine). Medication adherence should be an essential aspect of treatment for patients with METH-induced psychotic disorder after discharge from psychiatric wards. Changes in decision-making and cognitive impairment can lead to high recurrence rates among METH users (9, 10).
Studies have shown that locomotor activity is enhanced by cocaine and METH, suggesting a similar mechanism in exposure to these two drugs (11). Caffeine increases locomotor activity by inhibiting cGMP-preferring phosphodiesterase (PDE) at adenosine receptors, and paraxanthine is the active metabolite in this regard (12). Concomitant use of METH and caffeine also increases the symptoms of METH use (13, 14).
2. Objectives
Caffeine therapy is selected because of the following properties of caffeine or coffee usage: the consumption and use of caffeine are legal, and the product is widely accessible; it comes with no severe dependency and has low/no clinical or psychological complications post-consumption (15). Therefore, although some studies have been conducted to investigate the effectiveness of various medications on METH (modafinil, amantadine, amineptine, and mirtazapine), it should be noted that the search in all databases did not bring any findings in the form of articles or similar research work (16). Thus, the present study investigated caffeine's effects (Rescuecaf) on craving reduction and relapse prevention in METH use disorder.
3. Methods
3.1. Participants and Procedures
The statistical population of the present double-blind, randomized, placebo-controlled clinical trial was all people with METH use referred to the addiction treatment center of Qaemshahr in 2018. Seventy participants were randomly selected and assigned to the control (N = 35) and experiment (N = 35) groups. However, according to the exclusion criteria and participant dropout, the data obtained from 15 participants in each group were considered final data.
The inclusion criteria were METH-dependency based on the DSM-5, willingness and commitment to participate in the study, no severe illnesses, such as psychosis, major depression, tic disorder, and bipolar-polar disorder, no consumption of psychiatric medications, no sensitivity to stimulants, the absence of severe cardiovascular disease, no history of premature death due to cardiac failure in first-degree relatives, and no history of seizures and head trauma. Exclusion criteria were patients not following the medication protocol properly, severe side effects or severe medical disorder during the intervention, severe unstable vital signs, abnormal liver and kidney function tests, further METH use, and not continuing to participate in the study. Patients who had a proven history of clonidine, monoamine oxidase inhibitors, anticonvulsants, tricyclic antidepressants, and vasopressors usage were also excluded. More than one positive urine addiction test during the study was another exclusion criterion.
3.2. Allocation to the Experimental or Placebo Group
After implementing the inclusion and exclusion criteria, 30 patients were randomly assigned to the experimental and control (placebo) groups (N = 15). The categorization was done randomly using a simple decussate method, the first for the experimental and the next for the placebo, using a double-blinded status. Experienced clinicians and psychiatrists evaluated physical diseases, psychiatric disorders, inclusion and exclusion criteria based on the patient's medical records and counseling history.
3.3. Interventions
All patients received routine therapeutic counseling from an experienced psychologist. The patients were divided into two caffeine and placebo groups. Since the safe range of caffeine consumption is 1 - 9 mg/kg, the participants in the experimental group received 4.5 mg/kg (with an average daily dose of 300 mg) of sustained-release caffeine for three months. On the other hand, the placebo group was treated with tablets that shared similar properties in color and shape with caffeine tablets. The Noor Research and Educational Institute (TAVAN) provided the slow-release caffeine and placebo tablets. During the first week, the patients were interviewed daily through phone calls to be evaluated for side effects and addiction recurrence. Afterward, the screening program was performed weekly for up to three months.
3.4. Ethical Considerations
The test was formally listed on the Iranian Registry of Clinical Trials under the ID number IRCT2013120215 628N1, and the Ethics Committee of Baqiyatallah University of Medical Sciences approved the study (IR.BMSU.REC.1393.37). All human research procedures were consistent with the National Research Committee's ethical standards, the Helsinki Declaration of 1964, and its subsequent revisions or equivalent ethical norms. Informed consent was obtained, and the participants' information sheet recognized the participants' right to withdraw at any point during the research.
3.5. Measures
Researcher-made medication side effects questionnaire: This questionnaire includes questions about 36 common medication side effects. The questions were answered by participants as follows: 0 (not at all), 1 (a little), and 2 (much).
The Cocaine Selective Severity Assessment (CSSA) has 18 items scored on a 0 - 7 scale (0 = no symptoms and 7 = maximum severity) and assesses the severity of cocaine abstinence symptoms (17). Its score is obtained by summing the scores of all items. This scale measures symptoms and signs, such as cocaine craving, lethargy, appetite changes, depressed mood, sleep disturbances, and bradycardia commonly experienced following abrupt cessation of cocaine use. The instrument is a valid and reliable measure (17). Items with the highest scores scored by newly abstinent cocaine-dependent cases in the initial validity assessment of the scale are also the items of the DSM-IV diagnostic criteria for cocaine withdrawal: appetite changes, depressed mood (dysphoric mood), lethargy (fatigue), sleep changes, and irritability (psychomotor agitation). CSSA has excellent interrater reliability and internal consistency and is specific to cocaine dependence and decreases with the patient's continuous abstinence from cocaine within eight days. Initial CSSA scores are correlated with the frequency and amount of cocaine use and also measures of addiction severity from the addiction severity index (ASI) (17).
The Amphetamine Withdrawal Questionnaire (AWQ) is a self-completed instrument with ten items, which was developed based on the DSM-IV criteria for amphetamine withdrawal and measured the domains of craving, dysphoria, anhedonia, increased appetite, fatigue, agitation, anxiety, increased sleep, vivid, unpleasant dreams, and slowing of movement over the last 24 hours. Items are scored on a four-point Likert-type scale, from zero (not at all) to four (very much) (18). The possible range of scores is 0 – 40, with a higher score indicating greater severity. The validity of the Persian version of AWQ for questions individually and for the whole questionnaire was 80% and 86.5%. The internal consistency (Cronbach alpha) was 0.84 for the whole questionnaire (19).
Treatment adherence was determined based on the frequency of physician visits and counseling sessions attended by the patients. A urine test was performed weekly to screen the usage of METH, morphine, methadone, cannabis, and tramadol. Addiction severity was measured daily using the ASI (20). The rate of METH deprivation and the temptation to return were determined using the Visual Analogue Scale for stimulants.
The ASI has 106 items and six domains of medical status, drug use, employment status, family/social relations, legal status, and psychiatric status. In addition to the amount, this index measures the length and severity of drug use and the individual's status in occupational, family, legal, and psychological dimensions in the last 30 days. The items are scored on a five-point Likert-type scale (0 - 4). In this index, the medical, substance use, legal, occupational, family, and psychological status of drug addicts is assessed in the form of a clinical interview (21). Pearson's correlation coefficient showed a significant direct relationship between different dimensions of ASI and the intensity of consumption. Predictive validity was between 0.76 and 0.91, internal consistency with Cronbach's alpha method was between 0.65 and 0.89, and the concurrent validity of the test was 0.91 (22).
3.6. Data Analysis
Data were analyzed using SPSS 24 software for reporting descriptive statistics, comparing the data using repeated measure tests to assess the variable changes between steps of evaluations. Regression models and multivariate analyses of variance (MANOVA) were used to control the co-founding variables' impacts. The statistical significance level was considered at P < 0.05.
4. Results
Among all participants involved in the study (N = 70), 40 cases dropped out due to exclusion criteria, including stopping caffeine use, positive urine tests, and withdrawing from the study. Finally, data obtained from 30 participants in the experimental and control groups were analyzed. The mean age was 31.13 ± 3.06 years in the intervention group and 29.86 ± 4.64 years in the placebo group. Nine and seven participants were married in the intervention and control groups, respectively. Fourteen individuals in the experimental group and 12 cases in the control group held minimum high school diplomas. The duration of METH consumption was 3.26 ± 0.96 and 3.00 ± 1.85 years in the experimental and control groups, respectively. The sociodemographic characteristics of the participants in the study are presented in Table 1.
| Variables | Experimental | Control |
|---|---|---|
| Age | 31.13 ± 3.06 | 29.86 ± 4.46 |
| Duration of methamphetamine consumption | 3.26 ± 0.96 | 3 ± 1.85 |
| Marital status | ||
| Married | 9 (60) | 7 (46.66) |
| Single | 6 (40) | 8 (54.44) |
| Total | 15 (100) | 15 (100) |
| Level of education | ||
| High school diploma and higher | 14 (93.33) | 12 (80) |
| Under primary high school | 1 (6.66) | 3 (20) |
| Total | 15 (100) | 15 (100) |
a Values are expressed as mean SD or No. (%).
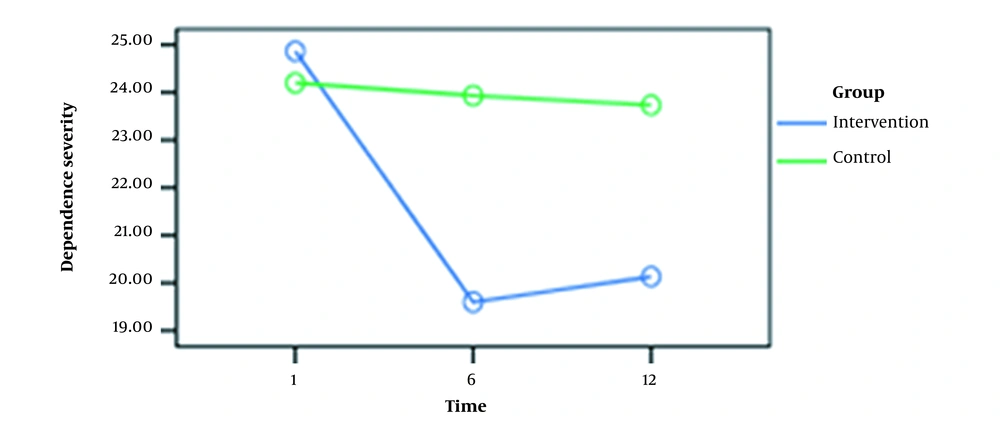
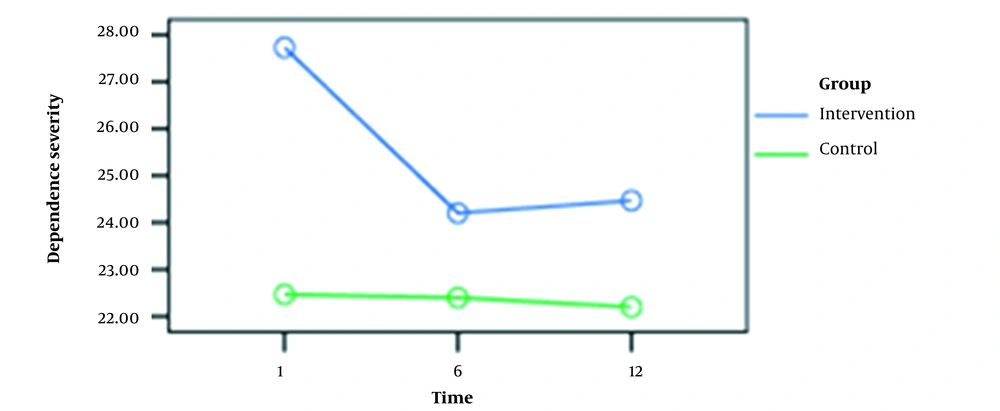
The mean scores of METH dependence and deprivation symptom severity are presented in Table 2.
| Variables and Groups | First Week | Sixth Week | Twelfth Week |
|---|---|---|---|
| Methamphetamine dependence severity | |||
| Caffeine | 24.86 ± 2.85 | 19.60 ± 2.09 | 20.13 ± 2.16 |
| Placebo | 24.20 ± 2.56 | 23.93 ± 2.40 | 23.73 ± 2.65 |
| Methamphetamine deprivation symptoms | |||
| Caffeine | 27.73 ± 4.62 | 24.20 ± 4.78 | 24.46 ± 4.74 |
| Placebo | 22.46 ± 3.75 | 22.40 ± 2.94 | 22.20 ± 3.38 |
a Values are expressed as mean ± SD.
Evaluation of METH dependency severity indicated no significant difference between the experimental and placebo groups. Nevertheless, the latest test rendered considerable results for the symptoms of METH deprivation (P > 0.05). Table 3 shows repeated measures for the severity of METH dependence and deprivation symptoms at three-time points (i.e., 1st, 6th, and 12th weeks).
| Variables | Total Squares | df | Mean Square | F | P-Value | Effect Size | Power |
|---|---|---|---|---|---|---|---|
| Methamphetamine Dependence Severity | |||||||
| Within groups | |||||||
| Time | 144.422 | 2 | 72.211 | 46.540 | 0.00 | 0.624 | 1.000 |
| Time/group | 109.356 | 2 | 54.678 | 35.240 | 0.00 | 0.557 | 1.000 |
| Error | 86.889 | 56 | 1.552 | _ | _ | _ | _ |
| Between groups | |||||||
| Group | 132.011 | 1 | 132.011 | 8.668 | 0.00 | 0.236 | 0.811 |
| Error | 426.444 | 28 | 15.230 | _ | _ | _ | _ |
| Methamphetamine Deprivation Symptoms | |||||||
| Within groups | |||||||
| Time | 63.622 | 1.161 | 54.783 | 13.850 | 0.00 | 0.331 | 0.968 |
| Time/group | 53.083 | 1.161 | 45.713 | 11.557 | 0.00 | 0.292 | 0.935 |
| Error | 128.622 | 32.518 | 3.955 | _ | _ | _ | _ |
| Between groups | |||||||
| Group | 217.774 | 1 | 217.774 | 4.741 | 0.03 | 0.145 | 0.557 |
| Error | 1286.178 | 28 | 45.935 | _ | _ | _ | _ |
As shown in Table 3, the effect of measurement time on METH dependence severity and METH withdrawal symptoms was significant between the intervention and placebo groups (F = 46.540 and F = 13.850, P > 0.05). In other words, this indicated that the differences in METH dependence and deprivation symptoms scores could vary depending on the measurement time and the assigned group.
As shown in Figures 1 and 2, there was a significant difference (0.05) between the mean scores at weeks 1 and 6 for dependence intensity and deprivation symptoms in the experimental group. However, no significant difference was observed between the mean scores at weeks 6 and 12. In the control group, these variables had no significant differences at weeks 1, 6, and 12.
| Phase A | Phase B | The Difference in Means of Phases A and B | P-Value |
|---|---|---|---|
| Methamphetamine Dependence Severity | |||
| First week | Sixth week | 2.76 | 0.00 |
| First week | Twelfth week | 2.60 | 0.00 |
| Sixth week | Twelfth week | - 0.167 | 0.99 |
| Methamphetamine Deprivation Symptoms | |||
| First week | Sixth week | 1.80 | 0.00 |
| First week | Twelfth week | 1.76 | 0.00 |
| Sixth week | Twelfth week | - 0.03 | 0.99 |
Bonferroni correction was used to assess the inter-group effect that showed significant differences in within-group factors (i.e., the time of measurement) and between-group variables (i.e., the group), based on the levels of the within-group factors (Table 4). The latest test revealed significant differences when comparing the severity of METH dependence and deprivation symptoms between the 1st vs. 6th and the 1st vs. 12th weeks. This does not hold for the 6th vs. 12th weeks in the caffeine group, indicating a long-lasting effect on both variables. When these variables were compared between the three-time points for the control group, pairwise comparisons did not show significant differences (Table 5).
| Complication | Intervention | Control | Second Week | Fourth Week | Sixth Week | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second Week | Fourth Week | Sixth Week | Second Week | Fourth Week | Sixth Week | χ2 | P-Value | χ2 | P-Value | χ2 | P-Value | |
| Decreased appetite | 15.3 | 14.1 | 16.4 | 15.7 | 16.8 | 14.5 | 0.02 | 0.87 | 1.25 | 0.26 | 0.56 | 0.45 |
| Weight gain | 12.7 | 12.5 | 13 | 18.3 | 18.5 | 18 | 3.4 | 0.06 | 3.5 | 0.06 | 2.9 | 0.08 |
| Dry mouth | 17.1 | 18.1 | 16.5 | 13.8 | 12.8 | 14.5 | 1.42 | 0.23 | 3.55 | 0.06 | 0.53 | 0.46 |
| Vomiting | 14.9 | 14.5 | 13.4 | 16.7 | 16.5 | 17.6 | 0.22 | 0.06 | 0.80 | 0.36 | 2.61 | 0.10 |
| Emotional instability | 16.3 | 15.7 | 16.8 | 14.6 | 15.3 | 14.2 | 0.37 | 0.53 | 0.02 | 0.89 | 0.77 | 0.37 |
| Sadness | 14.1 | 15.7 | 16.6 | 16.9 | 15.2 | 14.4 | 0.98 | 0.32 | 0.03 | 0.85 | 0.57 | 0.44 |
| Headache | 15.1 | 17.3 | 14.3 | 15.8 | 13.6 | 16.7 | 0.06 | 0.79 | 1.80 | 0.17 | 0.68 | 0.40 |
| Runny mouth | 16.3 | 15.2 | 15 | 14.7 | 15.7 | 15.9 | 0.32 | 0.57 | 0.03 | 0.85 | 0.11 | 0.73 |
| Acne | 15 | 15.4 | 14.9 | 16 | 15.5 | 16 | 0.23 | 0.63 | 0.03 | 0.95 | 0.22 | 0.63 |
| Nausea | 15.4 | 15.8 | 13.8 | 15.6 | 15.2 | 17.1 | 0.06 | 0.93 | 0.05 | 0.81 | 1.54 | 0.21 |
| Head lightness | 16.3 | 15.1 | 15.5 | 14.6 | 14.8 | 15.5 | 0.59 | 0.43 | 0.09 | 0.92 | 0.00 | 1.00 |
| Anger | 14.8 | 15.1 | 14.6 | 16.2 | 15.8 | 16.3 | 0.22 | 0.63 | 0.05 | 0.82 | 0.32 | 0.56 |
| Aggression | 15.5 | 15.5 | 15.5 | 15.4 | 15.4 | 17.4 | 0.02 | 0.96 | 0.02 | 0.96 | 1.75 | 0.18 |
| Diarrhea | 15 | 15.9 | 14.2 | 15.9 | 15 | 16.8 | 0.09 | 0.75 | 0.09 | 0.75 | 1.00 | 0.31 |
| Excess sweating | 15.5 | 15.1 | 15.5 | 15.4 | 15.8 | 15.5 | 0.05 | 0.98 | 0.06 | 0.79 | 0.00 | 1.00 |
| Weight loss | 15.8 | 15.8 | 14.9 | 15.1 | 15.1 | 16 | 0.07 | 0.79 | 0.09 | 0.75 | 0.22 | 0.63 |
| Stomachache | 14.4 | 15.5 | 14.6 | 16.6 | 15.4 | 16.3 | 0.58 | 0.44 | 0.02 | 0.96 | 0.38 | 0.53 |
| Limited excitement | 14.3 | 15.1 | 15 | 16.6 | 15.9 | 15.9 | 0.66 | 0.41 | 0.09 | 0.75 | 0.15 | 0.69 |
| Sleep problems | 17.5 | 14.1 | 16.3 | 13.4 | 16.9 | 14.6 | 2.00 | 0.15 | 0.95 | 0.32 | 0.35 | 0.54 |
| Irritability | 16 | 14.7 | 16.7 | 15 | 16.2 | 14.2 | 0.11 | 0.73 | 0.27 | 0.60 | 0.90 | 0.34 |
| Akathisia | 14.1 | 16.9 | 15.9 | 16.8 | 14.1 | 15 | 0.84 | 0.35 | 0.95 | 0.32 | 0.10 | 0.74 |
| Rush | 15.8 | 15.4 | 14.5 | 15.1 | 15.5 | 16.5 | 0.09 | 0.75 | 0.03 | 0.95 | 0.80 | 0.36 |
| Tic | 14.1 | 15 | 13.8 | 16.9 | 16 | 17.1 | 1.39 | 0.23 | 0.14 | 0.69 | 1.54 | 0.21 |
| Dizziness | 15 | 14.6 | 14.4 | 15.9 | 16.3 | 16.5 | 0.15 | 0.69 | 0.41 | 0.51 | 0.85 | 0.35 |
| Disinhibition | 14.6 | 15.4 | 14.9 | 16.3 | 15.5 | 16 | 0.41 | 0.51 | 0.03 | 0.95 | 0.22 | 0.63 |
| Fatigue | 13.3 | 15.2 | 15.7 | 17.6 | 15.7 | 15.2 | 2.48 | 0.11 | 0.03 | 0.95 | 0.03 | 0.95 |
| Loss of coordination | 14.9 | 15.3 | 14.5 | 16 | 15.6 | 16.5 | 0.22 | 0.63 | 0.01 | 0.91 | 0.80 | 0.36 |
| Paresthesia | 14.5 | 14.5 | 14.5 | 16.5 | 16.5 | 16.5 | 0.80 | 0.36 | 0.80 | 0.36 | 0.80 | 0.36 |
| Easy suffering | 16.8 | 13.4 | 16.2 | 14.2 | 17.5 | 14.7 | 0.79 | 0.37 | 2.02 | 0.15 | 0.24 | 0.61 |
| Tachycardia | 14.1 | 14.5 | 15.4 | 16.8 | 16.5 | 15.5 | 1.33 | 0.24 | 0.80 | 0.36 | 0.03 | 0.95 |
| Increased BP and PR | 14.1 | 15 | 14.1 | 16.8 | 16 | 16.8 | 1.33 | 0.24 | 0.23 | 0.63 | 1.33 | 0.24 |
| Gnashing teeth | 14.5 | 15 | 15.4 | 16.4 | 16 | 15.5 | 0.56 | 0.45 | 0.14 | 0.69 | 0.07 | 0.97 |
| Pallor | 14.3 | 14.9 | 14.6 | 16.7 | 16 | 16.3 | 0.76 | 0.38 | 0.22 | 0.63 | 0.41 | 0.51 |
| Rebound effect | 14.9 | 14.5 | 14.5 | 16 | 16.5 | 16.4 | 0.22 | 0.63 | 0.80 | 0.36 | 0.56 | 0.45 |
| Fear and anxiety | 15.7 | 14.2 | 16.7 | 15.3 | 16.8 | 14.2 | 0.01 | 0.89 | 1.00 | 0.31 | 0.84 | 0.35 |
| Other behavioral problems | 15.4 | 14.2 | 14.2 | 15.5 | 16.7 | 16.8 | 0.07 | 0.97 | 0.80 | 0.37 | 1.00 | 0.31 |
Based on the Kruskal Wallis test for 36 caffeine side effects, there were no significant differences between the experimental and control groups at the second, fourth, and sixth weeks.
5. Discussion
Relapse to substance use is a major health concern, and there is an urgent need for substance use treatments that maintain results over time. The present study aimed to explore caffeine (Rescuecaf) effectiveness in reducing craving and relapse prevention in METH dependence, which showed that METH dependence severity dropped significantly in the experimental group. However, this reduction was not significant in the placebo-treated patients. Also, similar findings were obtained for METH withdrawal symptoms.
No previous research on caffeine’s role in mitigating craving and relapse prevention in METH dependence was found in the review. However, in line with the results of this study, we can mention the role of neurophysiological variables and brain structures in drug use disorders and psychiatric disorders.
Amphetamine has stimulatory effects on the brain by activating depression-related neurons. It induces the secretion of serotonin, epinephrine, and norepinephrine in the brainstem and midbrain. These excitatory effects result in well-being and euphoria, cognition improvement, high energy, appetite suppression, and psychosis in long-term usage. Caffeine has similar effects on the brain. Some studies have reported the positive effects of low-dose caffeine on memory and cognitive skills. Caffeine is an adenosine antagonist. Adenosine is a modulatory agent in the nervous system with central excitatory and inhibitory effects. Caffeine populates adenosine receptors in the brain, which increases sympathetic activity. Thus, recent studies have demonstrated caffeine's effects on well-being, euphoria, and amphetamines. Using an agent with similar effects but less dependence helps patients overcome methamphetamine withdrawal symptoms (23). No specific drug has been approved to treat amphetamine dependence symptoms and physical and psychological manifestations of amphetamine withdrawal (24). One strength characterizing the present research is the assessment of caffeine side effects. We observed no significant difference when comparing the side effects in the two experimental and control groups.
Due to limitations, such as the lack of access to a larger community of men and women and the sample size, caution should be considered in generalizing the results of the present study. It should also be noted that the symptoms of high caffeine intake overlap with many mental disorders. Caffeine is influential in eating disorders, sleep disorders, and aggravation of anxiety and increases hostility and psychiatric symptoms (25). Thus, given the existing limitations, research in this regard should be conducted with larger sample sizes. In addition, using CSSA to measure METH severity can be another limitation in the present study.
5.1. Conclusions
Based on the results, caffeine is beneficial in controlling and ameliorating METH withdrawal complications without serious side effects. However, approving such therapeutic options demands further comprehensive clinical trials worldwide. Using the present study, the authors can state that caffeine as a new drug plays an influential role in managing craving reduction and relapse prevention in METH use disorder. It may enter the public pharmacopeia in the coming years after conclusive evidence.