1. Background
Attention deficit/hyperactivity disorder (ADHD) is the most common psychiatric disorder in childhood, with the prevalence accounting for 5 - 7% of the general population (1). This disorder might potentially continue during adolescence and adulthood at 70% and 50%, respectively (2).
Individuals suffering from ADHD face diverse socioeconomic difficulties with school, work, and family relationships and social interactions. They are at increased risk for perilous social behaviors, drug abuse, less schooling, and unemployment (3). Further investigations have shown that the concurrence of other psychiatric disorders, such as oppositional defiant disorder (67%), conduct disorder (46%), and anxiety (44%), is considerably high in ADHD (4).
Despite all efforts made to extensively study ADHD, this disorder still has a vast share of uncharted territories, including those regarding its pathogenesis and the mechanism of action of available pharmacotherapeutic agents, leaving ADHD patients vulnerable to challenges due to this lack of understanding (5).
Anxiety is one of the most significant conditions observed in ADHD cases. Although much is known about these two conditions when they occur independently, less is known about the comorbid conditions. The coincidence of comorbid conditions in ADHD children can induce greater impairments or unique treatment challenges compared to either condition alone (6). The treatment of ADHD is conventionally dependent on psychostimulants, including amphetamines and methylphenidate (MPH); nevertheless, the effects of these agents on comorbid anxiety in children remains a question (7). Other medications in this regard have not been well investigated; however, promising data have been presented for fluoxetine (FLX) use in ADHD patients with concurrent anxiety disorders (8, 9).
Another agent administered for this reason is atomoxetine (ATX), a highly selective inhibitor of the presynaptic norepinephrine transporter. Atomoxetine limitedly affects other transporters rather than norepinephrine, including serotonin or dopamine transporters, and has low affinity at dopaminergic, muscarinic-cholinergic, histaminic, serotonergic, and alpha1- or alpha2-adrenergic receptors (10). Studies in the literature have shown the well-tolerability of this agent for the treatment of ADHD; however, its anxiolytic and antidepressant effects on children have not been well-produced (6).
2. Objectives
This study aimed to evaluate the efficacy of the combination of MPH and FLX versus ATX in the symptoms and function of ADHD children with a concurrent anxiety disorder.
3. Methods
3.1. Study Population
The current randomized clinical trial was conducted on 76 children with a concurrent diagnosis of ADHD and anxiety disorder referring to outpatient psychiatry clinics of Khorshid and Amin hospitals affiliated with Isfahan University of Medical Sciences, Isfahan, Iran, from May 2020 to March 2021.
The study protocol was primarily designed according to the ethical tenets of the Helsinki Declaration proposed for the Ethics Committee of Isfahan University of Medical Sciences and approved based on the code number IR.MUI.MED.REC.1400.111. The present study was registered in the Iranian Registry of Clinical Trials and was accepted by the code number IRCT20211004052670N2. The legal guardians of the included population were informed about the study protocol, were reassured regarding the confidentiality of personal information, and signed written consent.
This study was performed on 6 - 12 -year-old children with a documented diagnosis of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5) who were recently diagnosed with a concurrent anxiety disorder using the DSM-5. The presence of any chronic disorder in the patient (i.e., neurological disorders, such as epilepsy, mental retardation, and cerebral palsy, and psychiatric disorders, including psychosis, mood disorders, bipolar disorders, diabetes mellitus, and any hematological or solid tumors requiring long-term medications that might have interactions with the medications) or a positive history of psychosis or mood disorders in the first-degree family members were considered the unmet criteria. Refusal to participate in or withdraw from the study, any new-onset neurological disorder in the period of the study, the incidence of significant drug-related adverse effects, and the advent of new psychological disorders, such as suicidal thoughts or attempts and agitation, were the exclusion criteria.
The patients who met the study criteria entered the study. They were assigned to one of the intervention groups through random blocks, each containing two members. Accordingly, a random alphabet letter from A - S and a random number (1 or 2) were allocated to each patient, determining their block using Excel Random Allocation Software (version 1.0). After joining the patients in the two-member blocks, the software was applied to determine whether blocks number 1 or blocks number 2 were assigned to each of the interventions (a or b). For instance, the patients were primarily allocated to one of the blocks A1 or A2, and then, the software determined who should apply MPH + FLX or ATX.
The present study was conducted in a double-blinded form as the person responsible for interviewing the patients and their parents and the statistician were blinded to the groups. The participants were enrolled in the study by a mentor who was aware that codes a and b indicated MPH + FLX and ATX, respectively. A biostatistician who was blinded to the medications performed all the randomizations. In addition, a psychiatry assistant who was blinded to the treatment of the patients interviewed the patients and their parents.
3.2. Interventions
The patients in the first group were treated with methylphenidate (Exir Pharmacy, Iran) plus FLX (Abidi, Iran). MPH was initiated with a daily dose of 5 mg twice daily. The dosage was increased to 10 mg weekly until the parents expressed their satisfaction with the treatment or significant adverse effects were noted. The maximal dose of MPH accounted for 60 mg per day. All the drugs were applied twice a day. Fluoxetine treatment was initiated with 2.5 - 5 mg daily with a weekly increase of 2.5 mg until achieving 10 - 20 mg per day based on the satisfaction of the psychiatrist with the response to the treatment. Fluoxetine was administered in the mornings. Fluoxetine treatment was begun at an interval of a week after methylphenidate.
The second group received atomoxetine (Tehran Darou, Iran) with an initial dose of 0.5 mg/kg, increasing to a dose of 1.4 mg/kg over a week. The drug was applied in the mornings. In cases with mild-to-moderate dyspepsia, the treatment with famotidine (Poursina Pharmaceutical Co., Iran) with a dose of 20 mg was prescribed. All the medications were applied for 4 months.
3.3. Assessment Instruments
Three questionnaires of the Conner’s Parents Rating Scale (CPRS), Screen for Child Anxiety Related Emotional Disorders (SCARED), and Children’s Anxiety Impact Scale-Child (CAIS-C) were used to assess the patients’ responses to the applied regimens. The assessments were carried out at the baseline and then repeated within 1 month and 4 months after the initiation of the interventions.
3.4. Conner’s Parents Rating Scale
Conner’s questionnaire contains 48 items assessing five groups of children with psychiatric disorders, including training disability, conduct problems, impulsive hyperactivity, somatic disorders, and anxiety. This scale is appropriate for 3 - 17-year-old children and has been designed to diagnose ADHD concurrent with other psychiatric disorders. The items are responded to by a 4-point Likert scale from 0 to 3. This questionnaire has been primarily validated with a Cronbach’s alpha of 0.90 (11). The Persian version of the CPRS has been validated by Shahim et al. with test-retest reliability and Cronbach’s alpha of 0.76 and 0.81, respectively (12).
3.5. Screen for Child Anxiety Related Emotional Disorders
The SCARED was designed by Birmaher et al. to assess the symptoms of anxiety disorders in 8-18-year-old children. This instrument consists of 41 items assessing panic disorder (13 items), generalized anxiety disorder (9 items), separation anxiety disorder (8 items), social panic (7 items), and school panic (4 items). Each item is graded by a 3-point Likert scale from 0 to 2 as incorrect, sometimes correct, and correct, respectively (13). The Persian version of this questionnaire has been validated by a Cronbach’s alpha ranging from 0.59 to 0.81 (14).
3.6. Children’s Anxiety Impact Scale-Child
The CAIS-C self-report questionnaire is a 27-item parent-and-child self-report questionnaire assessing the impact of anxiety symptoms on the psychosocial functioning of children and adolescents. The CAIS items are sorted into three categories of impairment in academic, social, and family environments. The parents and child respond to the parallel items scored on a 4-point Likert scale (“0” not at all, “1” just a little, “2” pretty much, and “3” very much). The total score comes out of the summation. This questionnaire has been validated with a Cronbach’s alpha ranging from 0.70 to 0.90 (15).
3.7. Outcomes
The primary outcome of this study was to assess and compare the efficacy of the interventions in the anxiety of ADHD patients using the CPRS, SCARED, and CAIS-C filled out at the baseline and within 1 month and 4 months after the interventions. Other retrieved data included demographic characteristics, including age and gender.
3.8. Statistical Analysis
The obtained data were entered into SPSS software (version 18). Categorical variables were presented in absolute numbers and percentages; nevertheless, continuous variables were expressed in mean ± standard deviation. The chi-square or Fisher’s exact test was applied to compare the categorical data. Continuous variables were compared using the independent t-test. Repeated measure analysis of covariance was utilized to assess the trend of changes in CPRS, SCARED, and CAIS-C scores. A P-value less than 0.05 was considered the level of significance.
4. Results
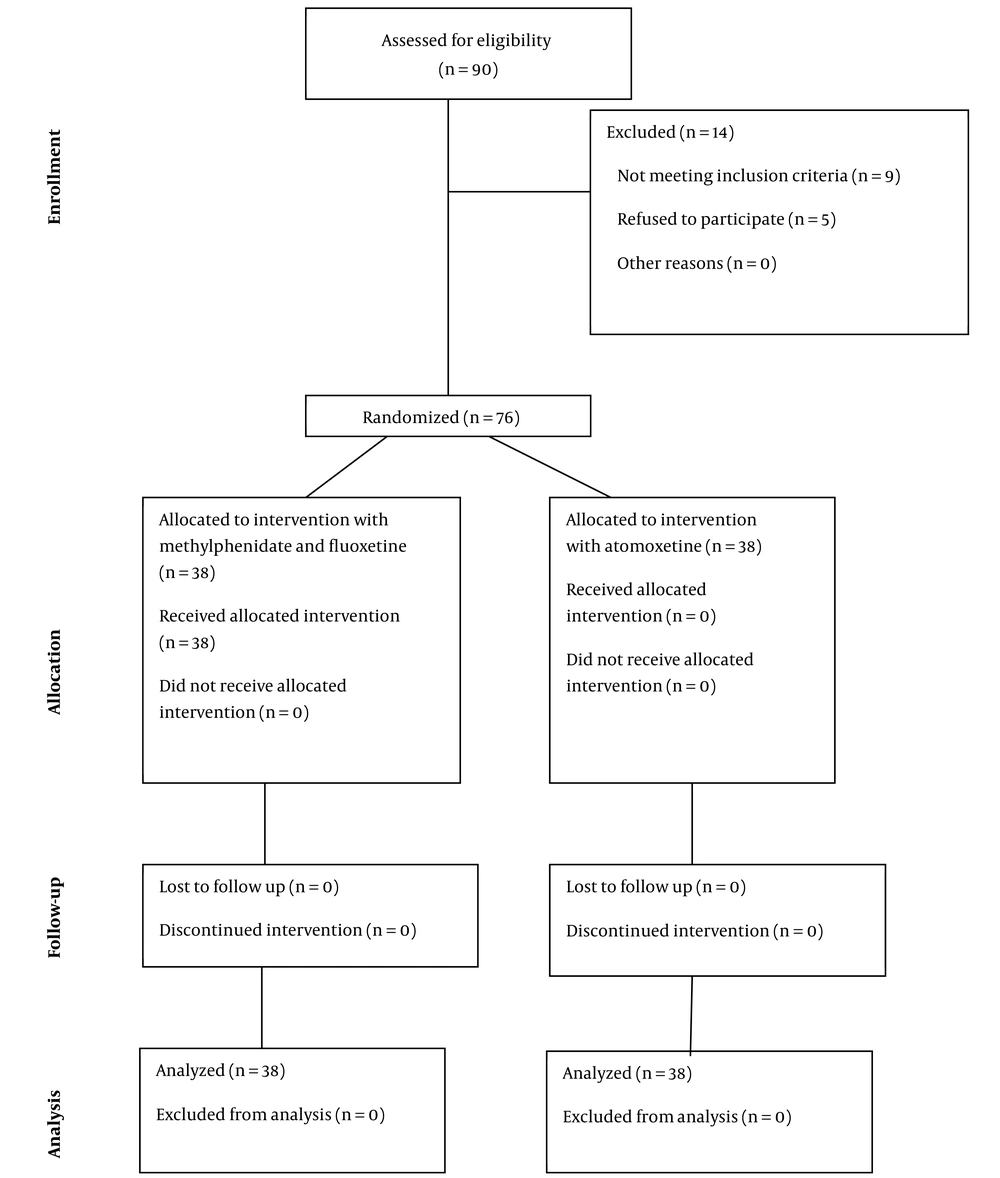
A total of 90 ADHD children with the probable diagnosis of anxiety disorders were primarily assessed regarding the eligibility of participation in the study, 76 cases of whom met the study criteria and were randomly allocated into groups of interventions (n = 38 for each group). None of the participants in the study group withdrew from the study. Finally, the current study was conducted on 76 ADHD children. Figure 1 depicts the CONSORT diagram of the studied population.
The mean age of the studied population was 9.26 ± 1.92 years, and 46 subjects (57.89%) were male. The two study groups were statistically similar in terms of age (P = 0.20) and gender distribution (P = 0.37) (Table 1).
| Variables | MPH + FLX (N = 38) | ATX (N = 38) | P-Value |
|---|---|---|---|
| Age (y) | 9.50 ± 2.08 | 9.03 ± 1.76 | 0.20 |
| Gender | 0.37 | ||
| Male | 23 (60.52) | 21 (55.26) | |
| Female | 15 (39.47) | 17 (44.73) |
Demographic Characteristics
Table 2 shows the trend of changes in the scores of the questionnaires applied to assess anxiety changes following the interventions. The baseline comparison of the scores revealed insignificant differences between the groups in all the assessments of the CPRS (P = 0.188), SCARED (P = 0.787), and CAIS-C (P = 0.680). Additionally, the investigations within 1 month and 4 months after the interventions revealed no difference between the groups in the assessments of the CAIS-C, CPRS, and SCARED (P > 0.05 for all). Further evaluations showed a significant trend of changes in both groups for all the scales of CAIS-C, CPRS, and SCARED in both groups (P < 0.001 for all the means of assessments and both the intervention groups). In general, the comparison of MPH + FLX efficacy versus ATX in anxiety in ADHD patients revealed insignificant differences regarding the scores of the CPRS (P = 0.397), SACRED (P = 0.663), and CAIS-C (P = 0.683).
| Variables And Timeline | MPH + FLX (N = 38) | ATX (N = 38) | P1 | P2 |
|---|---|---|---|---|
| CAIS-C | 0.397 | |||
| Baseline | 30.42 ± 9.52 | 29.78 ± 7.91 | 0.680 | |
| Within 1 month | 18.68 ± 7.16 | 18.74 ± 6.98 | 0.700 | |
| Within 4 months | 11.13 ± 7.62 | 10.39 ± 6.88 | 0.763 | |
| P3 | < 0.001 | < 0.001 | ||
| CPRS | 0.663 | |||
| Baseline | 89.86 ± 29.75 | 98.26 ± 29.77 | 0.188 | |
| Within 1 month | 47.89 ± 18.76 | 47.10 ± 17.59 | 0.811 | |
| Within 4 months | 27.61 ± 13.56 | 24.72 ± 11.59 | 0.485 | |
| P3 | < 0.001 | < 0.001 | ||
| SCARED | 0.683 | |||
| Baseline | 60.38 ± 23.76 | 61.88 ± 20.41 | 0.787 | |
| Within 1 month | 27.33 ± 18.27 | 25.90 ± 14.58 | 0.835 | |
| Within 4 months | 15.22 ± 14.18 | 11.50 ± 9.42 | 0.402 | |
| P3 | < 0.001 | < 0.001 |
5. Discussion
This study aimed to evaluate and compare the efficacy of two regimens, including the combination of PMH + FLX versus ATM, in anxiety disorders among children suffering from ADHD. As the demographic characteristics and baseline measurements were similar between the studied groups, all the outcomes can be attributed to the medical regimens. Accordingly, this study showed that both medications led to significant improvement in the anxiety scores of ADHD children; however, the comparison of the regimens revealed insignificant differences representing the similarity of ATX in comparison to MPH + FLX treatment.
Methylphenidate is the first-line well-known conventional treatment of ADHD mechanism of action which has been described in advance (16); however, considering the stimulant nature of MPH, its related side effects, including insomnia, decreased appetite, stomachaches, headaches, dizziness, irritability, anxiousness, and proneness to crying, must be precisely considered, particularly in those who have to administer various medications due to different disorders. A major body of evidence has presented dose-dependent side effects for MPH, magnifying the significance of applying the medications in the lowest doses causing the ultimate response and the least adverse effects (17).
Due to the significance of anxiety disorders among ADHD children, numerous efforts have been made to reduce the symptoms to improve the practice and quality of life in ADHD individuals (4). Fluoxetine is one of the old agents applied for this reason; however, a paucity of knowledge is available regarding the efficacy of its use (4). The most remarkable adverse effects of FLX include gastrointestinal and neurological symptoms; nevertheless, a major body of evidence has shown negligible side effects after FLX administration in ADHD. The other reason for favoring FLX refers to the long-term half-life leading to minimal required daily doses (18).
Gammon and Brown were the first group of researchers that applied FLX in combination with MPH to improve the response of ADHD children to medications and control mood disorders. Their promising outcomes (19) led to further investigations by Findling in 1996 that confirmed the significant improvement in mood disorders, such as anxiety disorders, among ADHD subjects who applied MPH plus FLX (20). The results of other studies regarding this issue are in line with the present study’s finding favoring adding FLX to MPH for the management of anxiety disorders in children suffering from ADHD (21, 22).
Moon et al. conducted an in-situ investigation assessing the effects of FLX plus MPH as an add-on therapy. They assessed dynorphin and substance P expression (both markers for striatal direct pathway neurons) and enkephalin (indirect pathway) by in-situ hybridization histochemistry. Their assessments showed that chronic MPH oral use alone leads to a tendency for a gradual increase in the expression of dynorphin and substance P expression; however, FLX alone could not affect gene expression. By the combined use of these agents, there was a significant improvement in the trend of dynorphin and substance P and, to a lower extent, encephalin. Therefore, they explained the action mechanism of this combination therapy and emphasized the value of their use to control ADHD-related symptoms, along with mood disorders (23). Another aspect that signifies the efficacy of this combination therapy relies on relatively fast drug absorption and high plasma levels (spikes) in contrast to using them lonely, factors critical for psychostimulant-induced gene regulation (24).
Atomoxetine has been applied for the management of ADHD since a long time ago (25). In addition, numerous studies in the literature have claimed its efficacy for controlling anxiety disorders in ADHD individuals alone or in combination with MPH (25-27); however, most of the authors insisted on better efficacy of ATX for controlling anxiety disorders than ADHD symptoms (27, 28).
The anxiolytic properties of ATX might be attributed to the central norepinephrine re-uptake inhibition. The other facet by which ATX affects anxiety refers to an autonomic function in the form of sympathetic tone attenuation, cardiac parasympathetic tone activation, and a decrease of sympathetic arousal to acute stress (29). Other factors favoring ATX use include not being a controlled substance and not having a significant impact on movement disorders. Neuroscience studies revealed that ATX increases dopamine in the prefrontal cortex, which is another condition relating to the ability to cope with anxiety (29). However, the potential adverse reactions of this agent should not be underestimated. Probably, the most significant trait of this agent refers to its metabolizing process through the cytochrome P450 2D6 (CYP2D6) enzymatic pathway. As the CYP2D6 has significant genetic polymorphism, the response to the medication might be variable. On the other hand, the concurrent use of medications inhibiting CYP2D6, such as selective serotonin reuptake inhibitors, can increase the serum levels of ATX. Nevertheless, this medication has been approved for children with ADHD; however, younger ages tolerate it better than adolescents (30).
Other adverse reactions to ATX include gastrointestinal symptoms, sleep disturbances (somnolence), cardiovascular adverse reactions, and other general disorders (e.g., irritability, dizziness, fatigue, and headache); however, a limited number of studies have mentioned suicidal ideation as one of the life-threatening adversities of ATX in children and young adults with ADHD (10).
The reason leading the authors to design this study is that up to 75% of ADHD children stop medication use in a period of time (31). Therefore, as anxiety disorders play a critical role in the performance of ADHD patients and their quality of life, decreasing the number of daily drugs that must be administered led to the conduction of the current study where ATX efficacy was equal to double therapy. This finding changed the opinions to using ATX rather than combination therapy.
5.1. Limitations
Along with the strength of this study, the small sample population, short term of the interventions, and inadequate dosage of each agent were the most significant limitations of the current study. Therefore, further studies are strongly recommended.
One of the overlooked points in the design of the current study is the application of a drug in one of the groups; nevertheless, the latter administered two drugs which might be a source of bias. However, the patients were categorized as groups a and b for the interviewer.
5.2. Conclusions
Based on the findings of this study, as ATX alone was as effective as MPH plus FLX for controlling anxiety disorders among ADHD children, ATX is preferred to apply fewer daily medications.