1. Background
Autism spectrum disorder (ASD) begins in early childhood and has short-term and long-term effects in later life. This disorder is characterized by deficits in social communication, social interaction, and limited and repetitive behaviors. According to updated data, ASD Global Prevalence is about 119 per 10000 (1). There is a geographical inequality in the prevalence of ASD, such that the Western Pacific and Eastern Mediterranean have the highest prevalence rates, respectively (1). In the eastern Mediterranean region, Iran is one of the countries where a high prevalence of autism has been reported (10 per 10000) (2).
Constellations of genetic, epigenetic, prenatal, perinatal, neonatal, and environmental risk factors have played a role in the etiology of ASD. These risk factors differ in the strength of association and evidence level for causality (3-5). Several important issues should be considered when judging the risk factors of an outcome. For example, the effect of a risk factor on an outcome may be different in the presence or absence of other risk factors, there are synergistic and antagonist relationships among risk factors and true associations would be identified when risk factors and outcomes are measured in the precise and consistent manner, etc. (6). Therefore, when we want to investigate the etiology of an outcome, it is better to measure all of the potential risk factors simultaneously with the minimum level of measurement error. However, it rarely happens that the majority of risk factors of ASD are measured together in a single study, and uniform observational data is available.
This gap can address by establishing a patient registry system. Through this system, uniform data on exposures and outcomes in a defined population would be collected. By conducting this study, researchers and policymakers can have the most up-to-date and accurate data on the burden of disease and its causes from a holistic perspective and then make an evidence-based decision about the best course of action (7).
Comprehensive registration can improve the better management and quality of health care. It can scientifically solve existing questions and raise new research questions (8).
A study has been conducted on developing an informative system of ASD Registry in Iran (9). However, the questionnaire questions in the study were incomplete, including the absence of all ASD risk factors based on the available literature, lack of educational and puberty characteristics, and lack of parental characteristics.
2. Objectives
Since valid and reliable data, as well as a sufficient sample size, are needed to identify risk factors and surveillance of ASD, we aimed to develop and design a registration system for ASD patients in Hamadan Province to have comprehensive and valuable epidemiological information for researchers about the frequency, distribution, and causes of ASD. This epidemiological information can help target prevention measures and plan care.
3. Methods
3.1. Study Design
The registry study is observational and prospective, including cases of ASD. The study population was patients with ASD in Hamadan province. We obtained the Ethical Code (Ref. No IR.UMSHA.REC.1401.422) from the Ethical Committee of Hamadan University of Medical Sciences. Enrollment and registration of cases will be begun in November 2022 until five years. The duration of the primary phase will be continuous, and over time, cases with ASD will be registered in this system. Written informed consent will be sought from all parents when necessary to allow sharing of anonymized data for approved research projects.
3.2. Case Definition
A case with ASD is defined as frequent difficulty communicating and interacting with others, restricted interests, and repetitive behaviors and symptoms based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5 criteria).
3.3. Data Collection Procedure
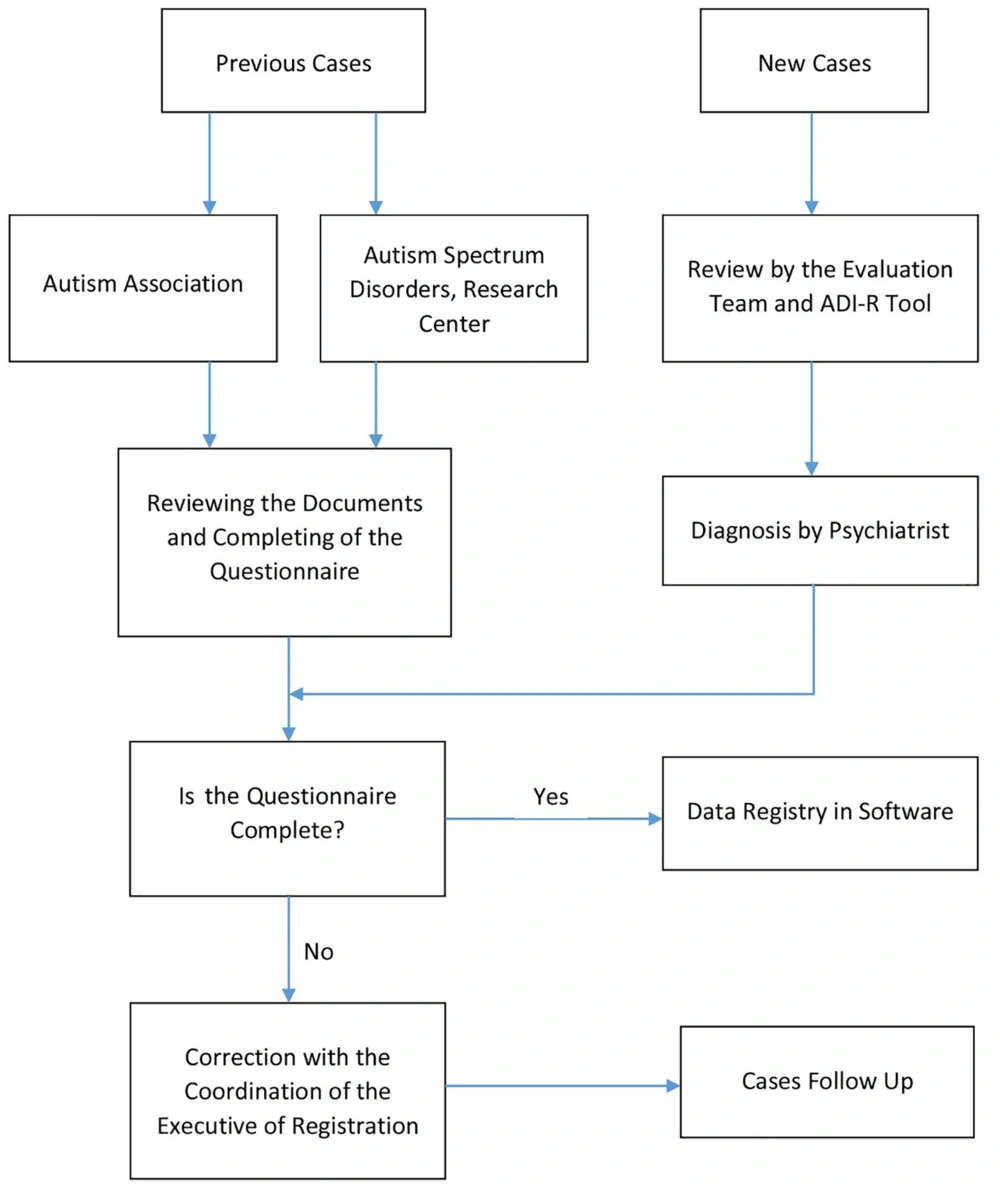
The cases that have already been diagnosed with ASD and have the documents in the Autism Association and Autism Spectrum Disorders Research Center, information will be entered into the registry system after reviewing the documents. All patients were diagnosed with ASD by a psychologist, and this diagnosis was confirmed by the autism diagnostic interview revised (ADI-R) tool. This tool was used for diagnosing autism, planning treatment, and distinguishing autism from other developmental disorders. The psychometric evaluation of this tool was performed by Bashirian et al. in Iran (10).
In new cases, if the child's healthcare provider or parents suspect a developmental problem, the child is referred to the Autism Spectrum Disorders Research Center. In addition, private psychiatric clinics and Farshchian Hospital refer cases with ASD to the Autism Spectrum Disorders Research Center to the registry and receive rehabilitation services.
Identification of cases with ASD is based on an evaluation team. The evaluation team will use observations, interviews with parents, and the ADI-R tool. Then the definitive diagnosis is the responsibility of the psychiatrist. After confirmation and diagnosis of ASD, the questionnaire will be completed for the case, and data will be entered into the software by a trained expert. The registered data is reviewed monthly to find defects. We will follow up on the incomplete questionnaires by the relevant expert to correct the data (Figure 1).
Regarding software, our registration system is written in PHP language, and its database is MySQL, and this system is designed as a web base. All participants will have access to their data and be trained in the use of available online data systems. Clinicians, researchers, and data entry personnel can access their local accounts with a username and password, and each user must change their unique password annually. Also, the research team is ready to cooperate with external organizations concerning data sharing within the framework of regulations.
There are protection rules in the field of system security that we define in this system for each user, a specific user, based on the defined roles; this user is only allowed to access the information entered by the person himself or the people who are defined for them. These people are defined through the system admin, and only the system admin, clinicians, and researchers have access to the data output. User access will be disabled if user information (user, password) is entered incorrectly 3 times, and access will be enabled again with the system administrator's permission. Data is backed up daily.
3.4. Measurement Tool
We will apply a researcher-developed questionnaire to collect data. To perform the validity, the questionnaire was designed by three authors. Subsequently, the questionnaire is reviewed and validated by an expert panel, including the principal investigator, psychologist, obstetrics, epidemiologist, occupational therapist, and speech and language therapist.
For comparability of results, we used DSM-5 codes for coding ASD. A dictionary will be used for the questionnaire to ensure uniformity in the register by the executive director. This questionnaire includes:
• Case characteristics (age, gender, blood group, residence, breastfeeding history)
• Educational characteristics (education, dropping out of school)
• Father characteristics (age, residence, job, physical and mental disorders)
• Mother characteristics (age, residence, job, physical and mental disorders)
• Pregnancy characteristics (unwanted pregnancy, infertility treatments, abortion, smoking, pregnancy complications)
• Delivery characteristics (delivery type, cesarean section reason)
• Neonatal characteristics (gestational age, multiparty, Apgar score, icterus, hypoxemia, neonatal weight)
• Puberty characteristics (age of puberty, menstruation for females)
• History of initial diagnosis (The age of the first concern of parents, Illness severity, use of diagnostic tools for ASD)
• Medical history and treatment procedures (sleep disturbance, sensory problems, urine, and stool control, The child's interactive situation, therapeutic measures)
3.5. Quality Control
The registration executive manager will review the completed questionnaires. If the questionnaire is incomplete, it will be referred again to the registration expert, who will contact the parents of the case and complete it. Each case will be entered into the software system based on the national code. Therefore, the registration software will not accept duplicate cases because they have the same national codes.
To ensure a correct diagnosis, the executive manager of registration will randomly review some of the ADI-R tools and the psychiatrist's diagnosis. To ensure compliance, the data will be entered into the software with the questionnaire; every week, random questionnaires of the cases registered by the registration expert will be checked by the executive director of registration.
Registration coverage of eligible cases will be estimated using percentages. Also, the frequency distribution of registered cases among different characteristics, including sex, age, and place of residence, will be estimated.
4. Conclusions
By designing and developing a patient registry system for ASD, valuable uniform epidemiological information about the web of causes and consequences of ASD is available. The next step is to utilize valid and reliable information for public health, clinical, or policy purposes.