1. Background
Borderline personality disorder (BPD) is characterized by disability in affect regulation, impulse control, self-image, and interpersonal relationships. Mood disorders, anxiety, substance abuse, attention deficit hyperactivity disorder, and cognitive impairments, such as executive functions, have been considered the most common comorbidities of BPD (1). Most patients have reported adverse life events during childhood, but the neurobiological mechanisms of BPD are poorly understood (2). The hyperactivity of the hypothalamic-pituitary-adrenal axis and impairment of serotonergic, glucocorticoid, aminergic, and glutamatergic systems might be involved in the pathophysiology of BPD (3).
N-Methyl-D-aspartate (NMDA) signaling pathways are critical in some neurodevelopmental alterations and neurological disorders (4). Over-activation of NMDA receptors following environmental stimulations, including chronic stress or social separation, involves the dysfunction of the hippocampus, amygdala, prefrontal, and cingulate cortex (5). High glutamate concentration in the anterior cingulate cortex exacerbates impulsivity and has been considered a diagnostic biomarker of BPD (6). Although psychotherapy is known as the primary treatment of BPD, some pharmacological treatments, including neuroleptics and selective serotonin reuptake inhibitors, have been prescribed to control severe symptoms (6). Furthermore, the improving effects of some NMDA modulators, namely lamotrigine, and topiramate, have been reported on anger, affective instability, and impulsivity of BPD (7).
Memantine, an uncompetitive voltage-dependent NMDA-receptor blocker, has been introduced as an effective medication to improve moderate to severe dementia in Alzheimer's disease (8). Some clinical trials have reported the potential efficacy of memantine in treating other neurological and neuropsychiatric disorders, such as peripheral neuropathy, depression, and schizophrenia (9, 10).
2. Objectives
A clinical trial in 2018 reported the improving effect of memantine at a dose of 20 mg/day on BPD symptoms (10). In that study, some patients had headaches, fatigue, or dizziness as the adverse effects of treatment. In our study, as the second clinical trial, to avoid any unwanted side effects, the efficacy of a low dose of memantine (10 mg/day) on the severity of BPD and the impairment of executive function was evaluated.
3. Methods
3.1. Ethics Approval
The Ethics Committee of Iran University of Medical Sciences (IR.IUMS.REC.1399.1185) approved the study protocol. After a complete explanation of the research process, written consent was obtained from the participants.
3.2. Participants
This randomized, double-blind, placebo-controlled clinical trial was performed on females or males aged 16 - 45 years referred to the Iran Psychiatric Hospital, Tehran, Iran (affiliated with Iran University of Medical Sciences, Tehran, Iran), and met the inclusion criteria. The sampling process was performed during Murch 2021-June 2022. BPD patients were diagnosed by psychologists based on the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for BPD. The participants were randomized, double-blind, and stabilized on medication and psychotherapy for at least four weeks.
The inclusion criteria were men and women aged 16 - 45 years, diagnosis of BPD, and ability to read and write in Persian. Exclusion criteria were neurological disorders, brain trauma, epilepsy, pregnancy, addiction, using drugs that might interact with memantine, and some psychotic conditions, such as schizophrenia, bipolar, psychotic depression, and mental retardation. Subjects who did not meet the mentioned conditions or were unwilling to complete a questionnaire were excluded.
Included participants were randomized by permuted block method and four blocks. In each block, 12 participants (six for the placebo group and six for the memantine group) were included and were matched in terms of three variables, age, gender, and education, with a ratio of 1:1. The outcome assessor, data analyzers, and randomizers were separate individuals, all of whom were blinded to allocation. The allocated group of each participant was printed sequentially and enveloped in a non-transparent and sealed envelope similar in appearance, using the random permuted block. The allocation was not within the reach of the subjects and outcome assessors. The outcome assessor, randomizer, and statistical analyzer were separate individuals, and all of them were blinded to allocation. Memantine and placebo tablets were similar in size, shape, color, and odor.
3.3. Interventions
Participants included in this study were divided into memantine (n = 20) and placebo groups (n = 19). Patients in the two groups were matched by age, gender, and education level. The details of the participants are shown in Table 1. Patients were stabilized on medication and psychotherapy in weeks 1 - 4 and then received a placebo or memantine (10 mg/day) in the placebo and memantine groups in weeks 5 - 8, respectively.
| Characteristics | Groups | Total | P-Value | |
|---|---|---|---|---|
| Placebo (n = 19) | Memantine (n = 20) | |||
| Age, mean ± SD | 27.42 ± 7.28 | 26.85 ± 8.63 | 27.12 ± 7.90 | 0.825 |
| Gender | 0.605 | |||
| Male | 12 (63.2) | 11 (55.0) | 23 (59.0) | |
| Female | 7 (36.8) | 9 (45.0) | 16 (41.0) | |
| Education level | 0.423 | |||
| high school diploma or less | 12 (63.2) | 15 (75.0) | 27 (69.2) | |
| University education | 7 (36.8) | 5 (25.0) | 12 (30.8) | |
| Marital status | 0.661 | |||
| Single | 16 (84.2) | 16 (80.0) | 32 (82.1) | |
| Married | 2 (10.5) | 4 (20.0) | 6 (15.4) | |
| Divorced | 1 (5.3) | 0 (0.0) | 1 (2.6) | |
| Employment | 0.658 | |||
| Employed | 4 (21.1) | 6 (30.0) | 10 (25.6) | |
| Unemployment | 14 (73.7) | 12 (60.0) | 26 (66.7) | |
| Homemaker | 1 (5.3) | 2 (10.0) | 3 (7.7) | |
| History of psychiatric disorders | 0.487 | |||
| No | 0 (0.0) | 2 (10.0) | 2 (5.1) | |
| Yes | 19 (100.0) | 18 (90.0) | 37 (94.9) | |
| Hospitalization history | 0.005 | |||
| No | 11 (57.9) | 3 (15.0) | 14 (35.9) | |
| Yes | 8 (42.1) | 17 (85.0) | 25 (64.1) | |
| Suicide attempts | 0.634 | |||
| No | 10 (52.6) | 9 (45.0) | 19 (48.7) | |
| Yes | 9 (47.4) | 11 (55.0) | 20 (51.3) | |
a Values are expressed as No. (%) unless otherwise indicated.
3.4. Outcomes
Changes in the severity of BPD symptoms and executive function as the main primary outcomes were assessed by the Borderline Evaluation of Severity Over Time (BEST) questionnaire and the Wisconsin Card Sorting Test (WCST), respectively.
The severity of BPD was assessed by the self-reported questionnaire named BEST. The acceptable BEST Persian version with high face and content validity and reliability has been published (11). Participants completed the BEST questionnaires in the baseline and weeks 2, 4, 6, and 8 of the trial. The BEST questionnaire contains fifteen items and three subscales, each scored from 1 to 5 on a Likert scale. The subscale A with eight items assesses thoughts and feelings during the past month. Subscale B, with four items, addresses negative behaviors during the past month. Items in subscales A and B are rated based on severity (1: None/slight; 5: Extreme). Subscale C with three items evaluates positive behaviors. The items in subscale C are rated based on their frequencies (1: Almost never; 5: Almost always). The total score of the severity of the disorder is obtained by subtracting the scores of subscale C from the sum of the scores of subscales A and B. The resulting scores are between -3 to 57. Finally, a correction factor of 15 is added to change the range to a positive direction. The final range of the scale is 12 - 72, which indicates BPD's low to high severity (12). The total score of the BEST difference between the baseline and weeks 2, 4, 6, and 8 among the two groups was the first outcome measured.
Moreover, the executive function of participants as the other outcome was measured by WCST. The WCST was performed at the baseline and end of the intervention (week 8) to assess executive function. This test was developed by Berg to evaluate flexibility in thinking and shifting to a new response to changing environmental contingencies (13). It is used as a measure of executive function (14). The WCST consists of four stimulus cards, and the subject receives two sets of 64 response cards. The subject should match response cards to the stimulus cards and receive feedback on whether he/she is right or wrong on each trial.
The computer-based Wisconsin test designed by the Sinai Research Institute of Cognitive Behavioral Sciences was used. The acceptable software of the Persian version with high face and content validity and reliability has been published (15). The participants were trained to match the suggested card to the four stimulus cards. The correctness or incorrectness of each trial was displayed on the monitor. The results of different subscales in WCST, including the number of wrong answers, perseverative errors, and categories achieved, were recorded (16). To assess the executive function of participants, each scale was compared at the baseline and the end of the trial in the placebo and memantine groups. Furthermore, side effects were assessed using a systematic questionnaire in both groups before and after medication administration.
3.5. Sample Size and Statistical Analysis
Based on the first clinical placebo-controlled trial published in 2018, the sample size with a power of 80% and a significance level of 5% was calculated (10). Assuming a 20% dropout rate, the sample size was 24 per group. The data were adjusted by the history of hospitalization and baseline variables as the main cofounders.
The comparison among categorical variables was made by the two-tailed Fisher’s exact test. The score changes in different time points within each group were analyzed by Repeated Measures ANOVA. Furthermore, the mean ± SD of the BEST score was compared at each time point between groups using the independent samples t-test. The probability values less than 0.05 were considered significant. The statistical software SPSS version 22 was used for data analysis.
4. Results
4.1. Participants
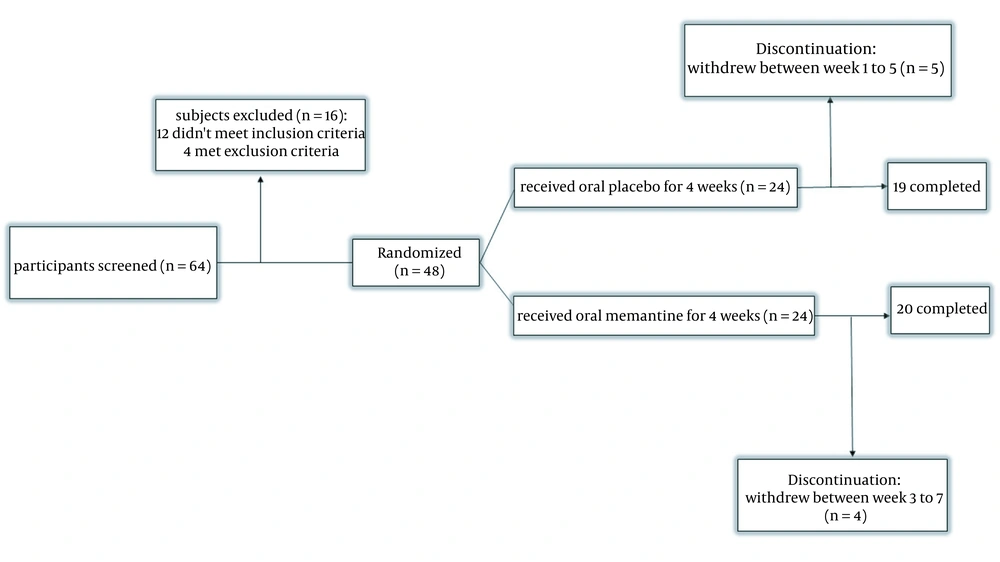
Sixty-four patients were enrolled in the study. Sixteen patients did not meet the inclusion criteria. Forty-eight participants were included and randomly divided into the placebo and memantine groups. Twenty patients in the memantine group and 19 patients in the placebo group completed the study (Figure 1). No one of the dropouts met adverse effects or drug interactions. The details of the characteristics are mentioned in Table 1.
The results of chi-square and Fisher's exact tests showed no statistically significant difference in the frequency distribution of variables (gender, education level, marital status, employment status, history of psychiatric disorders, and history of suicide attempts) between the two groups (P > 0.05). However, a significant difference was noted in the history of hospitalization (P = 0.005). In addition, the results of the two-sample independent t-test showed that the mean age was not significantly different between the two groups at a 95% confidence level (P = 0.825).
4.2. Adverse Side Effects
Adverse events were recorded during the study. Mild side effects were indicated in both placebo and memantine groups; however, they did not lead to treatment discontinuation. There was no significant difference in the frequency of side effects between the two groups (Table 2).
| Side Effects | Placebo, No. (%) | Memantine, No. (%) | P-Value |
|---|---|---|---|
| Somnolence | 1 (5.2) | 2 (10) | 0.82 |
| Tiredness | 2 (10.5) | 1 (5) | 0.83 |
| Headache | 0 (0) | 0 (0) | 0.99 |
| Decreased appetite | 1 (5.2) | 2 (10) | 0.82 |
| Vomiting | 0 (0) | 0 (0) | 0.99 |
| Dizziness | 0 (0) | 1 (5) | 0.85 |
| Diarrhea | 0 (0) | 0 (0) | 0.99 |
4.3. Severity Assessment of BPD
The mean total score of BEST was assessed in the placebo and memantine groups at different treatment time points (baseline, weeks 2, 4, 6, and 8; Table 3).
| Time Points | Placebo | Memantine | P-Value * | Adjusted P-Value $ |
|---|---|---|---|---|
| Baseline best | 39.26 ± 1.98 | 38.49 ± 3.39 | 0.390 | |
| Week 2 | 38.67 ± 2.78 | 37.13 ± 3.82 | 0.162 | 0.479 |
| Week 4 | 40.80 ± 3.65 | 39.46 ± 4.96 | 0.347 | 0.208 |
| Wee 6 | 40.35 ± 2.73 | 38.34 ± 4.44 | 0.098 | 0.426 |
| Week 8 | 39.76 ± 2.65 | 31.05 ± 4.07 | < 0.001 | < 0.001 |
| P-value # | 0.106 | < 0.001 |
a Values are presented as mean ± SEM. *, independent samples t-test; #, repeated measures ANOVA; $ analysis of covariance.
The mean ± S.E.M of BEST score significantly decreased after daily oral memantine received in week 8 compared to baseline (P < 0.001). There was no significant difference before and after the placebo administration. Moreover, the mean total score of BEST in the memantine group was compared to the placebo by independent samples t-test (Table 3). A significant reduction was indicated in the eighth week in the memantine group compared to the placebo group (P < 0.001). There was no significant difference between the groups on the baseline and weeks 2, 4, and 6.
The details of ANCOVA statistical analysis of unadjusted and confounder-adjusted estimates with 95% confidence interval are mentioned in Tables 4 and 5, respectively. The hospitalization history and baseline variables were adjusted by the cofounders.
| Parameter | B | Std. Error | t | P-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Week 2 | ||||||
| Intercept | 37.134 | 0.751 | 49.465 | <0.001 | 35.613 | 38.655 |
| Group | ||||||
| Placebo | 1.534 | 1.076 | 1.427 | 0.162 | -0.645 | 3.714 |
| Memantine | 0 | - | - | - | - | - |
| Week 4 | ||||||
| Intercept | 39.461 | .957 | 41.216 | < 0.001 | 37.521 | 41.401 |
| Group | ||||||
| Placebo | 1.337 | 1.372 | 0.975 | 0.336 | -1.442 | 4.117 |
| Memantine | 0 | - | - | - | - | - |
| Week 6 | ||||||
| Intercept | 38.350 | 0.813 | 47.161 | < 0.001 | 36.702 | 39.997 |
| Group | ||||||
| Placebo | 1.999 | 1.165 | 1.716 | 0.095 | -0.362 | 4.359 |
| Memantine | 0 | - | - | - | - | - |
| Week 8 | ||||||
| Intercept | 31.046 | 0.772 | 40.231 | < 0.001 | 29.482 | 32.610 |
| Group | ||||||
| Placebo | 8.718 | 1.106 | 7.885 | < 0.001 | 6.477 | 10.958 |
| Memantine | 0 | - | - | - | - | - |
| Parameter | B | Std. Error | t | P-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Week 2 | ||||||
| Intercept | 51.858 | 7.191 | 7.211 | < 0.001 | 37.258 | 66.457 |
| Baseline best | -0.350 | 0.185 | -1.888 | 0.067 | -0.725 | 0.026 |
| Hospitalization history | -1.535 | 1.200 | -1.279 | 0.209 | -3.972 | 0.902 |
| Group | ||||||
| Placebo | 1.179 | 1.165 | 1.012 | 0.479 | -1.185 | 3.543 |
| Memantine | 0 | - | - | - | - | - |
| Week 4 | ||||||
| Intercept | 18.235 | 9.094 | 2.005 | 0.053 | -0.227 | 36.697 |
| Baseline best | 0.520 | 0.234 | 2.221 | 0.033 | 0.045 | 0.995 |
| Hospitalization history | 1.496 | 1.518 | 0.986 | 0.331 | -1.585 | 4.578 |
| Group | ||||||
| Placebo | 1.529 | 1.473 | 1.038 | 0.347 | -1.461 | 4.519 |
| Memantine | 0 | - | - | - | - | - |
| Week 6 | ||||||
| Intercept | 28.256 | 8.159 | 3.463 | 0.001 | 11.692 | 44.821 |
| Baseline best | 0.245 | 0.210 | 1.167 | 0.251 | -0.181 | 0.671 |
| Hospitalization History | 0.808 | 1.362 | 0.593 | 0.557 | -1.957 | 3.573 |
| Group | ||||||
| Placebo | 2.133 | 1.321 | 1.614 | 0.098 | -0.549 | 4.816 |
| Memantine | 0 | - | - | - | - | - |
| Week 8 | ||||||
| Intercept | 40.981 | 7.639 | 5.365 | < 0.001 | 25.474 | 56.489 |
| Baseline best | -0.226 | 0.197 | -1.148 | 0.259 | -0.625 | 0.173 |
| Hospitalization history | -1.497 | 1.275 | -1.174 | 0.248 | -4.086 | 1.091 |
| Group | ||||||
| Placebo | 8.271 | 1.237 | 6.686 | < 0.001 | 5.759 | 10.782 |
| Memantine | 0 | - | - | - | - | - |
a The hospitalization history and baseline variables have been considered cofounders.
4.4. Wisconsin Subscale Scores
Wisconsin subscales, including the number of wrong answers, perseverative errors, and categories achieved, were compared in each group on the baseline and week 8 (beginning and end of the trial) (Table 6). There was no significant difference in none of the wrong answers and categories achieved subscales before and after medication administration in the placebo and memantine groups. However, perseverative errors significantly increased in the memantine group before and after medicine administration (P < 0.05). The details of ANCOVA statistical analysis of unadjusted and confounder-adjusted estimates with 95% confidence interval are mentioned in Tables 7 and 8, respectively. The hospitalization history and baseline variables were adjusted as cofounders.
| Wisconsin Subscales | Placebo | Memantine | P-Value * | Adjusted P-Value $ |
|---|---|---|---|---|
| Wrong answers | ||||
| Before | 30.11 ± 10.00 | 36.70 ± 3.44 | 0.012 | |
| After | 32.00 ± 9.63 | 33.80 ± 8.92 | 0.549 | 0.222 |
| Difference between before and after | 1.89 ± 5.34 | -2.90 ± 9.14 | 0.053 | |
| P-value # | 0.140 | 0.172 | ||
| Perseverative errors | ||||
| Before | 9.84 ± 6.62 | 4.70 ± 3.95 | 0.005 | |
| After | 9.00 ± 6.86 | 7.70 ± 7.22 | 0.284 | 0.118 |
| Difference between before and after | -0.84 ± 2.67 | 3.00 ± 5.97 | 0.007 | |
| P-value # | 0.186 | 0.037 | ||
| Achieved categories | ||||
| Before | 2.53 ± 1.90 | 3.85 ± 1.87 | 0.017 | |
| After | 2.89 ± 2.18 | 3.20 ± 2.50 | 0.344 | 0.160 |
| Difference between before and after | 0.37 ± 1.67 | -0.65 ± 2.76 | 0.720 | |
| P-value # | 0.35 | 0.114 |
a Values are presented as mean ± SEM. *, independent samples t-test; #, paired samples t-test; $, analysis of covariance.
| Parameter | B | Std. Error | t | P-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| After Treatment, Wrong Answers | ||||||
| Intercept | 33.800 | 2.074 | 16.297 | 0.000 | 29.598 | 38.002 |
| Group | ||||||
| Placebo | -1.800 | 2.971 | -0.606 | 0.548 | -7.821 | 4.221 |
| Memantine | 0 | - | - | - | - | - |
| After Treatment Achieved Categories | ||||||
| Intercept | 3.200 | 0.526 | 6.080 | 0.000 | 2.134 | 4.266 |
| Group | ||||||
| Placebo | -0.305 | 0.754 | -0.405 | 0.344 | -1.833 | 1.223 |
| Memantine | 0 | - | - | - | - | - |
| After Treatment, Preservative Error | ||||||
| Intercept | 7.700 | 1.576 | 4.886 | 0.000 | 4.507 | 10.893 |
| Group | ||||||
| Placebo | 1.300 | 2.258 | 0.576 | 0.284 | -3.275 | 5.875 |
| Memantine | 0 | - | - | - | - | - |
| Parameter | B | Std. Error | t | P-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| After Treatment, Wrong Answers | ||||||
| Intercept | 10.361 | 7.290 | 1.421 | 0.164 | -4.438 | 25.160 |
| Before right answers | 0.720 | 0.168 | 4.284 | 0.000 | 0.379 | 1.062 |
| Hospitalization history | -3.528 | 2.824 | -1.249 | 0.220 | -9.262 | 2.206 |
| Group | ||||||
| Placebo | 1.437 | 2.981 | 0.482 | 0.222 | -4.614 | 7.488 |
| Memantine | 0 | - | - | - | - | - |
| After Treatment Achieved Categories | ||||||
| Intercept | 0.539 | 1.085 | 0.496 | 0.623 | -1.665 | 2.743 |
| Before achieved categories | 0.816 | 0.163 | 5.000 | 0.000 | 0.484 | 1.147 |
| Hospitalization history | -0.564 | 0.697 | -0.808 | 0.425 | -1.980 | 0.852 |
| Group | ||||||
| Placebo | 0.533 | 0.701 | 0.759 | 0.160 | -0.891 | 1.957 |
| Memantine | 0 | - | - | - | - | - |
| After Treatment, Preservative Error | ||||||
| Intercept | 2.498 | 1.866 | 1.338 | 0.189 | -1.291 | 6.286 |
| Before preservative error | 0.952 | 0.154 | 6.183 | 0.000 | 0.640 | 1.265 |
| Hospitalization history | 0.854 | 1.893 | 0.451 | 0.655 | -2.989 | 4.698 |
| Group | ||||||
| Placebo | -3.231 | 2.016 | -1.603 | 0.222 | -7.324 | 0.862 |
| Memantine | 0 | - | - | - | - | - |
a Hospitalization history and baseline variables were considered cofounders.
5. Discussion
In this double-blind, placebo-controlled clinical trial, we examined a low dose of memantine (10 mg/day) for eight weeks to improve BPD symptoms. Our data showed a significant decrease in the mean score of BEST on week 8 compared to weeks 2 and 4 in the memantine group. In addition, a significant decrease in this score was indicated in the memantine group compared to the placebo group on week 8.
The therapeutic effect of memantine on BPD was in the same direction as other different psychiatric disorders. Based on reported studies, memantine prevented stress-induced problems and caused mood stabilization in bipolar disease (17-19). Moreover, memantine could diminish disinhibition, irritability, aggression, and false impression in Alzheimer's disease (20). Irritability in patients with autism and mania in patients with bipolar disorder were significantly improved by memantine (21, 22). In addition, the efficacy of memantine at a higher dosage (20 mg/day) has been reported to improve BPD symptoms; however, more than 40% of participants experienced some adverse effects, including mild headache, fatigue, or dizziness (10).
In this regard, our data showed that a low dose of memantine (10 mg/day) for 8 weeks could effectively improve BPD symptoms while the side effects of the medication were decreased. However, the participants were not matched in terms of hospitalization history, and the data were adjusted by this confounder variable. More participants with a history of hospitalization were included in the memantine group indeliberately. Although the history of hospitalization might be related to the severity of the disorder, the relation between the history of hospitalization and the severity of the disorder was not assessed in our study. This bias has been carried out unintentionally, and more assessment would be necessary in future studies.
In a 12-week study on memantine monotherapy in adults with ADHD with 10 mg daily, some participants had mild adverse effects, such as systolic blood pressure and mood and visual problems (23). Therefore, a follow-up longer than 8 weeks in future studies seems to help determine the presence or extent of side effects at a low dose (10 mg/day) of memantine in BPD patients.
Executive function deficits are one of the most common cognitive problems among BPD patients and are associated with self-harm behaviors, impulsivity, and social functions (24-26). Memantine has been reported to ameliorate cognition disturbances in Alzheimer's, epilepsy, and breast cancer (11, 27). On the other hand, it fails to improve cognition in Parkinson's disease or Down syndrome (28). In the current study, we evaluated the effect of memantine on the cognitive impairments of BPD subjects, and our results showed that memantine could not improve cognitive executive disabilities. Some research indicated that a dose-dependent steady-state plasma level of memantine is needed to achieve desirable cognition (29, 30). Therefore, to achieve a potential improvement in executive cognitive functions in BPD patients. The prescription of memantine for more extended periods is needed in future studies. Moreover, we only used WCST to examine the executive function and cognition of our participants. Perhaps, the assessment of different subdomains of executive functioning through related tests, namely the listening span task (LST), Eriksen flanker task (FT), or letter fluency task (LFT) (31), in future studies can be helpful to gain more precise results. In addition, the inclusion of more participants with a history of hospitalization in the memantine group, which might indicate a more severe disorder, might be involved in the lack of effectiveness of memantine in WCST. Therefore, matching the participants regarding hospitalization history in future investigations is recommended.
Memantine, at a higher dosage, has affected serotonin, sigma-1, and nicotinic acetylcholine receptors, as well as serotonin and dopamine uptake. Lower dosage administration acted as an NMDA receptor blocker, especially in the CNS (32). It has been indicated that the hypo-activation of NMDA receptors triggered neural toxicity that might originate from GABAergic neurons disinhibition since diazepam and barbiturates suppressed ketamine-induced psychosis (32). The neurotoxicity severity depends on the type and dosage of NMDA receptor antagonist (33, 34). Memantine, as a low-affinity antagonist, had fewer side effects than other NMDA receptor blockers. In addition, our findings proposed that a low dose of memantine administration might reduce the probability of general side effects occurrence.
In conclusion, memantine as an NMDA antagonist seems to be effective in improving some symptoms of BPD (35). Our findings suggested that a low dose of memantine might be considered a new pharmacological approach to improve some BPD symptoms without adverse effects.
5.1. Limitations
Some limitations in our research should be considered in future studies. Considering delayed response in some psychiatric disorders, and individual differences in response to medications, a longer follow-up than 8 weeks seems to be beneficial for achieving stronger and clear efficacy of treatment on the severity of symptoms and cognitive functioning of patients (36). Moreover, we only used WCST to examine the executive function and cognition of our participants. Using more tests to examine different aspects of cognition can further strengthen and clarify the results. In addition, matching the participants in terms of hospitalization history should be considered in the future.