1. Background
Depression is one of the most debilitating disorders in the world today. It is estimated that by 2030, it will become the second most costly disorder in the world (1). The etiology of this disorder is based on multiple factors that may operate at different biological, genetic, psychological, and social levels. For this reason, the mechanisms involved in symptoms and their complex relationships have not yet been fully identified (2).
Various experimental studies indicate that facial feedback can moderate experienced emotional states and create new emotions (3-6). This finding includes more details, especially regarding anger (7) and happiness (8). Also, there are findings on sadness and fear(9, 10), as well as surprise and disgust (11). The facial feedback hypothesis suggests that changes in facial muscle states occur voluntarily and automatically. For instance, voluntary smiles are generated in the motor cortex and through the extrapyramidal motor system, while involuntary smiles are mainly created through subcortical nuclei and the extrapyramidal nervous system. This implies that producing and maintaining an emotional state can induce corresponding emotions (12). Recently, two new bodies of research have improved our knowledge about the mechanisms of facial emotional feedback and have taken steps toward explaining the clinical benefits of facial muscle manipulation of corrugator and zygomaticus muscles in depression.
Studies have explored the effects of Botox injections in the corrugator muscles to alleviate depressive symptoms. Functional MRI results indicate that people with deactivated corrugator muscles due to Botox injections exhibit less amygdala activity when expressing anger than those without such injections (13). Also, results indicate that even when facial muscles were not active, the feedback led to changes in mood through sensory nerves of the skin (14) or signals related to the perception of the muscle's state in subcutaneous nerves (15, 16). Overall, multiple studies suggest that the inactivation of the corrugator muscles through Botox injections has significant anti-depressant effects compared to a placebo (17-21). On the other hand, some evidence suggests that electrical stimulation of facial muscles may positively impact mood and depression (1, 22). Functional electrical stimulation (FES) uses electrical stimuli to produce muscle contractions and induces activity-dependent plasticity in brain neural circuits. It is used to maintain or restore neuromuscular functions during/after disuse or improve neuromuscular functions in healthy individuals (23). Although functional electrical stimulation for these purposes is still under investigation and requires further research to establish, there is some research on the use of electrical stimulation of facial muscles to reduce depressive symptoms and improve mood. Zariffa et al. (22) performed functional electrical stimulation on zygomaticus muscles to increase positive mood in depressed patients. The results showed that after a single session of electrical stimulation, the individuals in the experimental group performed significantly better than the control group in components such as determination, courage, and concentration. Additionally, Kapadia et al. (1) demonstrated that functional electrical stimulation of zygomaticus major muscles and orbicularis oculi muscles significantly reduced the severity of depression and improved sleep patterns and parameters.
Although various forms of facial muscle stimulation have been used to reduce or change negative mood, all studies on the effect of Botox in reducing depression have focused on the corrugator muscles. In contrast, all studies on electrical stimulation of facial muscles have sought to create facial feedback through the zygomaticus muscles. Theoretically, we can assume that electrical stimulation of cranial facial muscles (such as frontalis muscles) can reduce negative mood through reducing activity in the corrugator muscles. Electric stimulation of facial muscles can affect the areas of the central nervous system associated with emotions, such as the amygdala and basal ganglia. Studies have shown that this can reduce connectivity between the amygdala and the anterior cingulate cortex, resulting in a reduced ability of the cortex to regulate subcortical areas in mediating negative emotions, commonly referred to as deficits in "top-down" control (24). As a result, electrical stimulation of facial muscles can potentially reduce negative mood by modulating limbic system activity through the facial nerves.
2. Objectives
Considering the two research studies mentioned above, it is evident that all the interventions to investigate the effect of functional electrical stimulation on depression have only focused on the zygomaticus muscles, which are involved only in positive emotions. Also, although the effectiveness of corrugator muscle inactivation through botulinum A injection has been shown in various studies to reduce the severity of depressive symptoms, as far as the authors of this article know, the effectiveness of functional electrical stimulation of frontalis muscles (involved in negative affect) in decreasing depressive symptoms has not yet been examined. Therefore, the present research tried to implement facial feedback through electrical stimulation of the facial muscles and investigate its effect on the type and extent of changes related to emotion, mood, and cognitive-emotional variables in people with depression. Our goal in this study is to determine the effects of bilateral electrical stimulation of the frontalis (as inhibitory muscles of the corrugator) and zygomaticus muscles on the emotions and mood of people with mild depression.
3. Methods
3.1. Design
This study utilized a multiple baseline single-subject design with a one-month follow-up period to examine the effects of facial electrical stimulation on participants' zygomaticus or frontalis muscles. This design is appropriate to investigate the impact of a single intervention and is particularly useful when there are at least three participants (25). This study had three baseline periods of three, six, and nine days, with two participants in each period. Electrical stimulation was administered to the zygomaticus muscles for participants 1, 2, and 3 and to the frontalis muscles for participants 4, 5, and 6 over 28 sessions. The study also included a follow-up period to assess the durability of the intervention effect. The interventions were staggered across different tiers to establish experimental control, plotting each tier on a separate graph. The interventions were introduced systematically in one tier following a clear procedure. Meanwhile, the other tier did not receive any interventions, but they continued to collect baseline data from the participants. This process continued until all the conditions completed the AB sequence, meaning they had data from the baseline (A) and intervention (B) phases.
3.2. Participants
Through purposive sampling, six subjects with mild depression were selected who met the inclusion criteria. They were asked to fill out Beck's Depression Inventory (BDI) during psychiatric interviews to determine the severity of their depression. Those who scored 14 to 20 on the BDI were considered mildly depressed (26). They met the following criteria: (1) age of 23 - 42 (27), (2) no significant changes in their drug regimen or any other form of therapy at least six weeks before the intervention (1), (3) score of 14 - 22 on the Beck's Depression Inventory (26), (4) no alcohol, substance, or drug use (1), (5) no history of brain injuries, epilepsy, or seizures (1), (6) no history of severe mental illnesses, such as schizophrenia or bipolar disorder (22), (7) no medical history of using antipsychotic drugs and opioid blockers (1), (8) no history of any dysfunction or paralysis in facial muscles (27), (9) no history of electrical stimulation therapy on their faces and neck (1), (10) no current pregnancy (1), and (11) no current comorbid disorders like OCD and anxiety disorders based on the psychiatric interview (28).
3.3. Procedure
The study's sampling, intervention, and data gathering were conducted in 2022. Six individuals were referred to the rehabilitation centers affiliated with the University of Rehabilitation Sciences and Social Health, Tehran, Iran. They were selected based on their severity of depressive symptoms according to clinical interviews (conducted by Ph.D. holders in clinical psychology) and BDI (29). Finally, they were assigned to the triple series. After the clinical interview, the participants were asked to fill out BDI as part of the clinical observation. To account for baseline variability, the participants were randomly divided into two groups and underwent frontalis or zygomaticus muscle stimulation. Due to the homogeneity of demographic characteristics among the subjects, we randomly assigned participants into two groups using a computer program that generated a sequence of numbers. Each participant was matched with a number in the sequence. We followed a simple rule to assign the participants into two groups: Odd numbers were assigned to the frontalis stimulation group, and even numbers to the zygomaticus stimulation group. Thus, participants 1, 3, and 5 were in the frontalis group, and participants 2, 4, and 6 were in the zygomaticus group. This method ensured a random and unbiased assignment. In the second step, we randomly selected pairs consisting of one number from each group and placed them in each baseline. After baseline assessment (positive affect and negative affect) and pretest implementation to assess the level of depression, mood, and cognitive ability (selective attention and processing speed) for all 6 participants, according to the baseline of 3, 6, and 9 days, the participants entered the intervention phase of the study. After the final session, a posttest was conducted on the participants. The follow-up assessments were carried out one month after the participants' post-test. The study was approved by the Ethics Committee of the University of Social Welfare and Rehabilitation Sciences (IR.USWR.REC.1400.121).
3.4. Intervention
The Lift Plus an electrical muscle stimulation device was used in this study. Lift Plus is a portable, battery-powered device with two positive and negative ball electrodes. This device has a voltage of 10 to 90, stimulating the target muscles by creating asymmetric wave fields. Lift Plus has four treatment programs having intensity levels from 1 to 20. Increasing the electrical stimulation intensity increases muscle traction significantly. The parameters involved in the first electrical stimulation program of Lift Plus included 2-second rhythmic electrical field stimulation with 3-second intervals at a frequency of 40 Hz (27). The intervention was implemented over 28 days for each participant (one intervention session a day). Each intervention session lasted for about 30 minutes, which included an assessment of affect and mood (15 to 20 minutes), performing electrical stimulation of the muscles on one side of the face, rest, and performing electrical stimulation of the muscles on the opposite side of the face (10 to 15 minutes).
The experimental sessions were held in the emotional-cognitive laboratory of the clinical psychology group at the University of Rehabilitation and Social Health Sciences. In the intervention stage, the participant received a description of the experimental setting based on a specific script when they came to the laboratory. They were told their participation was voluntary and asked to read and sign the consent form. The participant saw the areas of the face under stimulation. Before the experiment, the participant went through a pre-interview and completed questionnaires. The moderator also demonstrated how the stimulation devices worked and let the participant practice the stimulation on their hand skin. The participant's face was cleaned with freshen-up towels to prepare for the experiment. The participant used the Lift Plus device to stimulate specified muscles on both sides of the face. The participant began with the minimum intensity level and gradually increased until the stimulated muscle showed noticeable movement. The participant could use a mirror, and the moderator verified the movement. Each of the device's ball electrodes had conductive gel on the surface before the stimulation began. The participant could apply the more conductive gel on the ball contacts if they felt discomfort during the stimulation. The final option was to remove the device from the treated area.
3.5. Measures
3.5.1. Beck's Depression Inventory (BDI-II)
It is one of the most commonly used self-assessment scales for depression, with 21 items. In 1996, Beck designed this inventory to determine the symptoms of depression symptoms' severity. Today, BDI-II is widely used for clinical and experimental purposes. The BDI has been validated in Iran with test-retest reliability of 0.93 and convergent validity between BDI and the Beck Hopelessness Scale, Suicide Ideation Scale, and Hamilton Depression Rating Scale.
3.5.2. The Positive and Negative Affect Scale (PANAS)
It is a self-assessment tool with 20 items to assess two aspects of one's mood, positive and negative affect (23). Each subscale has 10 items. The participants answer these items on a 5-point Likert scale. Since one's affective states are subject to constant changes, in this study, positive and negative effects were examined daily immediately before the intervention sessions. The internal reliability of the measure used with an Iranian population was found to be very good, with a Cronbach's alpha coefficient of 0.87
3.5.3. The Digit Span Subset of The Wechsler Adult Intelligence Scale (WAIS-IV)
This scale examines the short-term memories of the participants. Furthermore, the Digit Span subset can provide valuable information about participants' ability to maintain their attention. The higher scores on the Digit Span subset indicate a strong working and short-term memory, which, in turn, suggests one's ability to sustain one's selective attention.
3.5.4. The Symbol Search Subset of The Wechsler Adult Intelligence Scale (WAIS-IV)
In this test, the participant must write the code designed for each digit on a specific paper based on the instructions they received. After practicing for a short while on some sample tests, the participant is given 90 minutes to write as many codes as possible about the digits in a form. In this subset, factors include the ability to learn new tasks, visual motor speed, perseverance, and processing speed. A study investigated the test-retest reliability of the Farsi The Wechsler Adult Intelligence Scale (WAIS)-R and found high levels of reliability for all subtests and the total scale (ranging from 0.82 to 0.96) (30)
3.5.5. The Visual Analogue Scale (VAS)
It is a visual analog scale to assess one's mood. It is designed on a Likert scale in which the participants, based on the visual data provided for them within the scale, describe their mood states in a range from 0 (elevated mood) to 10 (deflated mood) (24). The Visual Analogue Scale (VAS) scores were examined every two days before the intervention sessions because the nature of one's moods is sustainable over a long period.
3.6. Statistical Analysis
Several measures were utilized to evaluate changes in the participant's affective states during 28 intervention sessions and generate visual representations of the data. We employed Cohen's effect size, mean percentage improvement (MPI) for desired behavior, mean percentage reduction (MPR) for an unwanted behavior, percentage of non-overlapping data (PND) for increased desired behavior, and percentage of overlapping data (POD) for decreased unwanted behavior. The PND and POD are sensitive to level changes, while alterations influence percentage decrease and improvement in trend line slope. Cohen's d is sensitive to variability as defined by standard deviation. A linear mixed model analysis was also implemented to compare study phases and identify reliable changes from pre-intervention to post-intervention. The AB design is a common single-case experimental design utilized in clinical research (31). It comprises two distinct phases, usually a baseline and a treatment phase, and can be perceived as an interrupted time series (Campbell & Stanley, 1966). Appropriate analytical techniques are essential to ensure an adequate assessment of the variances between the baseline and treatment phases in this type of interrupted time series. A linear mixed model can be used in single-subject case studies to examine the effect of an intervention over time while accounting for individual variability and potential confounding factors. This statistical method allows for incorporating both fixed and random effects and can handle missing data and unequally spaced time points. Using a linear mixed model, researchers can obtain a more comprehensive understanding of the intervention's impact on the individual case and potentially identify factors that moderate or mediate the intervention's effectiveness (32). For this purpose, we utilized the widely-used software package SPSS, specifically version 20 (SPSS, 2011), to assess if there was a significant difference in the changes in measures during the intervention compared to the baseline.
4. Results
This section will begin by presenting sociodemographic information about the participants in the study. Subsequently, we will provide a visual and statistical analysis of the data acquired from the research in two subsections. Table 1 displays the demographic and clinical characteristics of the participants.
| Variables | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|
| Gender | F | F | M | F | F | M |
| Age | 23 | 30 | 33 | 37 | 24 | 24 |
| Marital status | Single | Single | Married | Single | Single | single |
| Hand dominance | R | R | R | R | R | R |
| Medication | SSRI's | None | SSRI's | SSRI's | SSRI's | SSRI's |
| Baseline score on BDI | 21 | 22 | 19 | 17 | 21 | 20 |
| Location of stimulation | Zygomaticus | Zygomaticus | Zygomaticus | Frontalis | Frontalis | Frontalis |
Abbreviation: BDI, Beck's Depression Inventory.
4.1. Visual Inspection
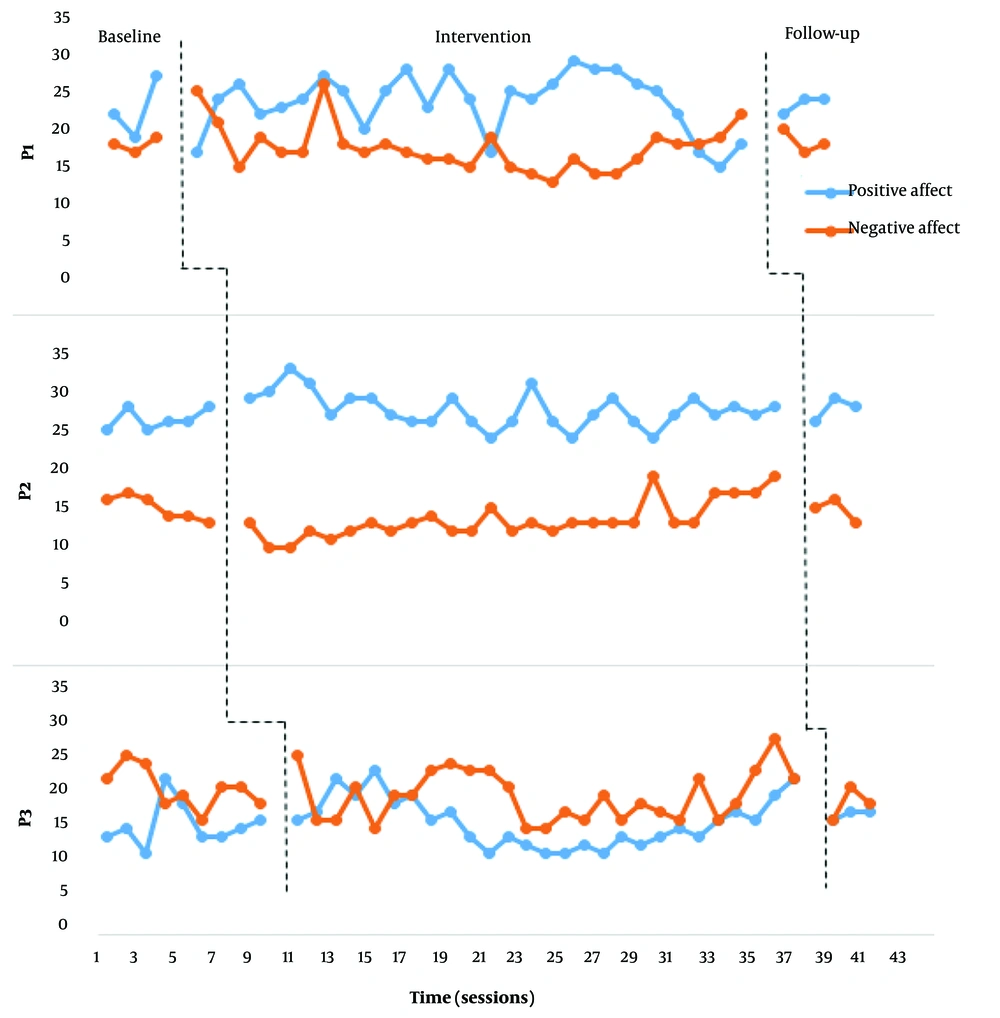
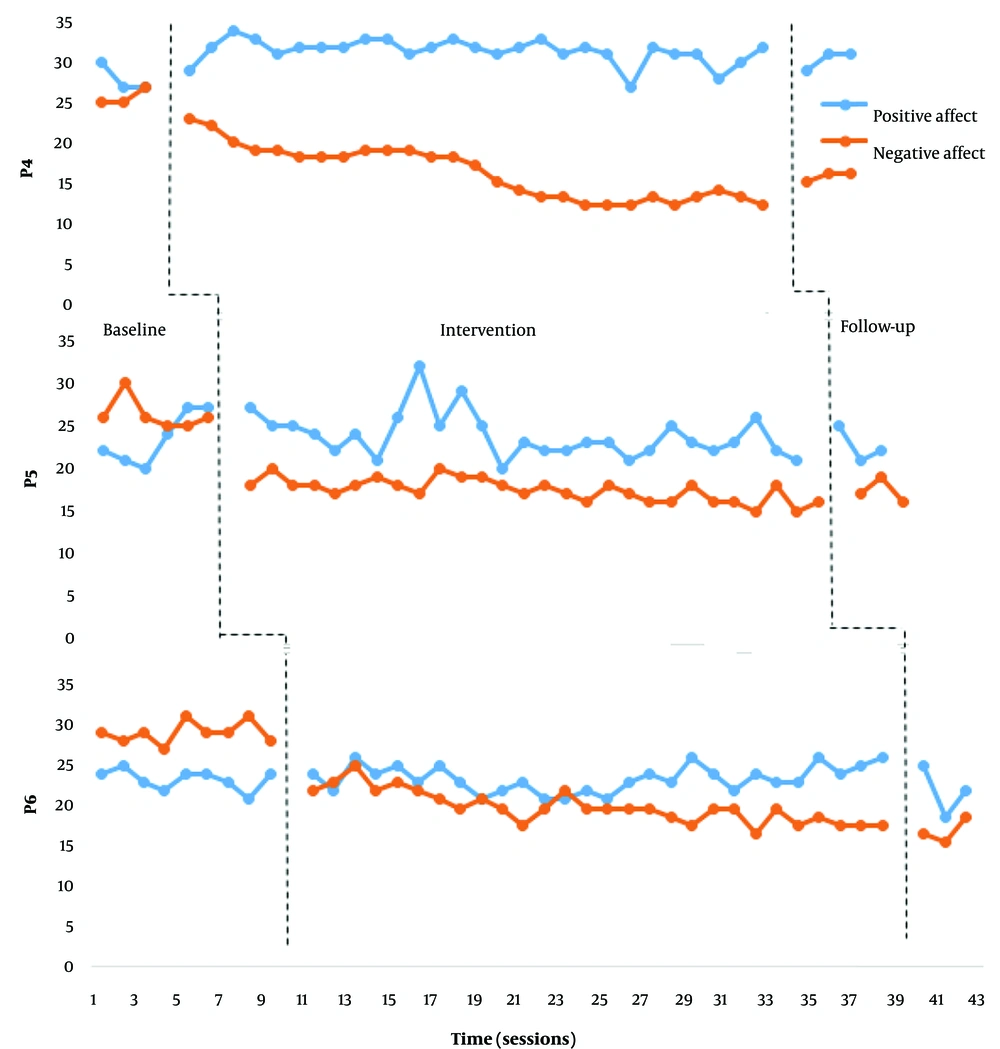
Figures 1 and 2 demonstrate the positive and negative affect scores of each participant in the baseline, intervention, and follow-up stages.
According to Figures 1 and 2, none of the participants showed a significant change in their positive affect scores. Furthermore, no substantial changes in the negative affect scores of participants 1, 2, and 3 were observed. However, we observed a significant decrease in the negative affect scores of participants 4, 5, and 6 (frontalis muscles electrical stimulation). After the intervention phase, the mean percentage reduction (MPR) for participants 4, 5, and 6 were 62.3, 49.52, and 46.29. And Cohen's d, respectively, 3.95, 4.47, and 5.62.
We observed relative and absolute level changes in participants 4, 5, and 6 from the initial sessions. From sessions 13 to 15, while observing a downward trend in the negative effect, we noticed a relative fluctuation in the scores. Moreover, from session 15 onwards, while maintaining the downward trend, the negative effect in all three participants showed a decreasing pattern. At the follow-up stage, the scores of participants 5 and 6 remained relatively unchanged compared to a month ago. Participant 4 demonstrated a relative increase in negative affect compared to the last intervention session. However, the variability level at the follow-up stage of this participant was still at an acceptable level compared to the baseline stage. It indicated that the participant was on the path to recovery. The decreased percentage of negative affect for each of the three participants was highly significant.
4.2. Comparisons Across Study Phases
Pre-treatment to post-treatment changes were analyzed using a mixed model analysis. All observation scores of the treatment (28 sessions) and baseline phases were included in this analysis for positive and negative affect. Also, mixed model analysis was used for pre-intervention and post-intervention of the VAS, BDI, digit span, and symbol search scores (pretest and posttest). Pre-intervention (mean scores), post-intervention, and follow-up scores on standardized measures, along with the results of the Wilcoxon signed-rank test for determining significant differences between post-treatment and follow-up scores, are presented in Table 2.
| Measure | Baseline (M) | Post-treatment (M) | One Month Follow-up (M) | Wilcoxon Signed-rank Test (P) |
|---|---|---|---|---|
| Depression | ||||
| Z stimulation | 20.66 (1.52) | 17.33 (1.52) | 17.66 (0.57) | 0.90 |
| C stimulation | 19.33 (2.08) | 10 (2.00) | 9.66 (3.21) | 0.95 |
| Mood | ||||
| Z stimulation | 5.70 (0.61) | 4.35 (1.06) | 5.58 (0.19) | 0.250 |
| C stimulation | 7.05 (0.78) | 4.96 (0.45) | 4.99 (0.87) | 0.750 |
| Selective attention | ||||
| Z stimulation | 11.33 (1.53) | 12 (2.64) | 11.33 (2.30) | 0.50 |
| C stimulation | 11 (1.00) | 11.66 (0.57) | 12 (1.00) | 0.90 |
| Processing speed | ||||
| Z stimulation | 49.66 (6.42) | 54.66 (4.93) | 54 (5.29) | 1.00 |
| C stimulation | 46 (3.46) | 58.33 (1.52) | 58 (3.51) | 0.520 |
The results of the mixed model analysis are included in Table 3. The parameters and P values presented in Table 3 determine the slope change and the significance of the slope change in the treatment period compared to the baseline. These parameter estimates can also be interpreted as effect size (33).
| Estimate | SE | P | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Positive affect | ||||||
| Z stimulation | Time in phase * Phase | 0.7453 | 0.71 | 0.302 | 0.69 | 2.18 |
| F stimulation | Time in phase * Phase | -0.6618 | 0.41 | 0.121 | -1.50 | 0.18 |
| Negative affect | ||||||
| Z stimulation | Time in phase * Phase | 0.4039 | 0.4534 | 0.378 | -0.57 | 1.31 |
| F stimulation | Time in phase * Phase | 1.3566 | 0.1491 | 0.019 | 0.06 | 0.65 |
| Mood | ||||||
| Z stimulation | Time in phase * Phase | 1.2566 | 0.3862 | 0.047 | 0.02 | 2.48 |
| F stimulation | Time in phase * Phase | 2.0900 | 0.4290 | 0.017 | 0.72 | 3.45 |
| BDI (depression) | ||||||
| Z stimulation | Time in phase * Phase | -3.3333 | 1.2472 | 0.056 | -0.12 | 6.79 |
| F stimulation | Time in phase * Phase | 9.3333 | 1.6666 | 0.005 | 4.70 | 13.96 |
| Digit Span | ||||||
| Z stimulation | Time in phase * Phase | -0.6666 | 1.7638 | 0.725 | -5.56 | 4.23 |
| F stimulation | Time in phase * Phase | -0.6666 | 0.6666 | 0.374 | -2.51 | 1.18 |
| Symbol Search | ||||||
| Z stimulation | Time in phase * Phase | -5.0000 | 4.6785 | 0.345 | -17.98 | 7.98 |
| F stimulation | Time in phase * Phase | -10.3333 | 0.8819 | 0.000 | -.12.78 | -7.88 |
Abbreviation: BDI, Beck's Depression Inventory.
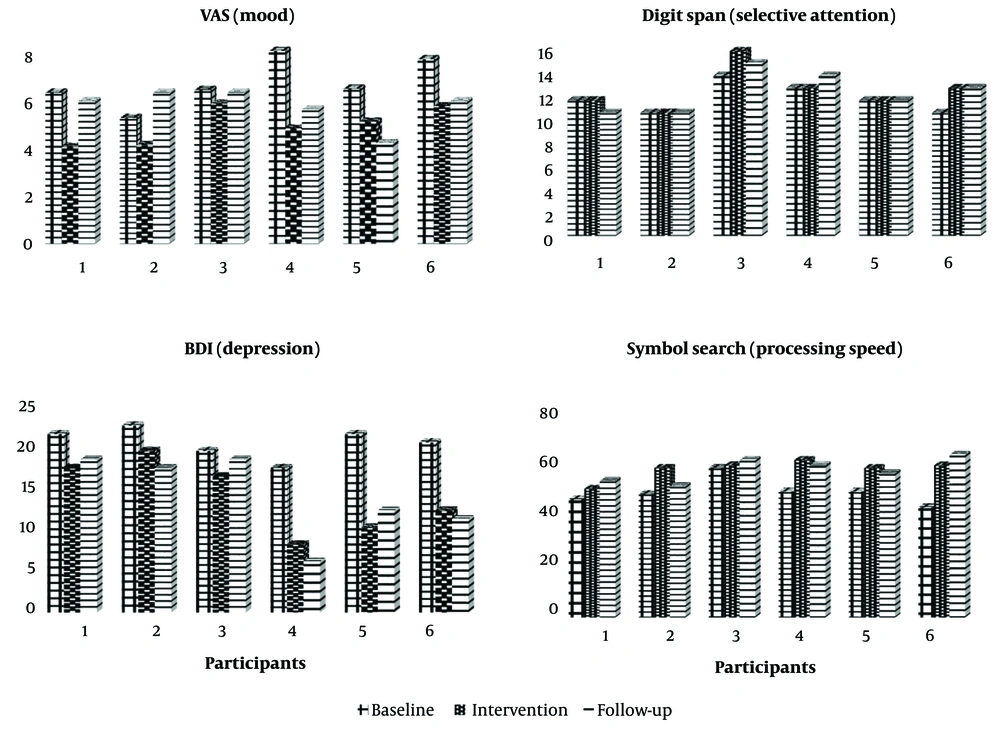
Regarding differences in trends between the treatment and baseline phases, negative affect (P = 0.019), mood (P = 0.017), depression (P = 0.005), and symbol search (P < 0.01) were improved in participants with frontalis muscle stimulation, and mood (P = 0.047) was improved in participants with zygomaticus muscle stimulation. Significant estimations in this table show meaningful slope changes in the results of the intervention phase compared to the baseline. Figure 3 depicts all participants' VAS, BDI, Digit Span, and symbol search scores at the baseline, intervention, and follow-up stages.
The visual data in Figure 3 clearly show the changes in the dependent variables of depression, mood, processing speed, and selective attention across all six participants. Participants 4, 5, and 6 showed significant changes from baseline to the post-intervention phase, and these changes in the follow-up phase were maintained to a large extent. Also, fixed effects of mixed model analysis results indicate that there was no significant change in the scores of depression (F: 0.023; P = 0.886), mood (F: 0.118; P = 749), and processing speed (F: 154; P = 715) in participants 4, 5, and 6 during the follow-up stage compared to the post-intervention stage. Additionally, although the mood scores of participants 1, 2, and 3 increased compared to post-intervention, the changes were not statistically significant (F: 6.30; P = 0.066). Based on the results, it can be concluded that the changes observed in the scores from the post-intervention stage to the follow-up stage appear to be relatively stable. Also, according to Table 2, no significant difference was observed between follow-up and post-intervention scores of depression, mood, processing speed, and selective attention.
4.3. Clinically Significant Change
Based on Jacobson's model of clinical significance (34), the BDI was used in this study. At the intervention and follow-up stages (one month after the last intervention session), all 3 participants receiving the frontalis muscles electrical stimulation met the Segger et al. standardized recovery criteria with a reliable change index of 8.46 (35). A qualitative analysis of the depression scores of the frontalis muscle's electrical stimulation indicates a significant difference (9, 11, and 8 points) in the post-intervention phase. These scores indicate a significant decrease in depression post-intervention compared to the baseline. Based on the cutoff scores of the BDI (36), these scores indicate a significant decrease in depression scores in mildly depressed individuals to the degree that they were no longer considered clinically depressed. The differences in the BDI scores from the baseline to the follow-up stage for participants 4, 5, and 6 were 8, 11, and 10.
5. Discussion
We aimed to examine the effectiveness of emotional feedback through the electrical stimulation of facial muscles in decreasing the severity of depressive symptoms in mildly depressed individuals. According to the literature, this study hypothesized that the electrical stimulation of the caudal (zygomaticus) and cranial (corrugator) muscles of the face, depending on the point of stimulation, will improve one's mood and decrease depressive symptoms by affecting the negative and positive affect.
The findings of this study indicate that the bilateral electrical stimulation of the zygomaticus muscles has no significant impact on increasing positive affect, decreasing negative affect, improving one's mood, and alleviating depressive symptoms. While the existing literature on this topic is not substantial, the findings of this study fall in line with the findings of Zariffa et al. (22), who discovered that while the two-way electrical stimulation of the zygomaticus muscles increases some components of positive affect (courage and concentration), this intervention has no significant impact on the overall positive and negative affect. It is worth noting that electrical stimulation interventions in this study were conducted within one session for each participant. On the other hand, Kapadia et al. (1) discovered that the simultaneous functional electrical stimulation of zygomaticus and orbicularis oculi muscles decreases depression severity and sleep patterns and parameters significantly. However, no significant changes in the positive and negative affect were observed in this study. We could not highlight the effectiveness of electrical stimulation of the zygomaticus muscles in decreasing the severity of depressive symptoms and improving one's mood. However, what sets our study apart from Kapadia et al. (1) is the electrical stimulation of orbicularis oculi muscles responsible for spontaneous smiles. This factor can explain why the results of our study differed from the previous studies. Therefore, it can be concluded that the electrical stimulation of zygomaticus muscles alone might not increase the positive effect and alleviate depressive symptoms. Furthermore, according to the findings of this study, the bilateral stimulation of the frontalis muscles can decrease negative affect, improve one's moods, and reduce depressive symptoms significantly. The findings of this study, in line with the findings of a group of studies, show that the deactivation of forehead corrugator muscles can decrease the negative effect and severity of depressive symptoms (37-39).
It is discovered that the formidability of the frontalis muscles as the result of constant electrical stimulation can decrease forehead muscles' tractions. Also, deactivating these muscles through Botox injections decreases negative emotions significantly. Two important hypotheses are introduced on the inhibitory impact of muscle tractions in decreasing the negative effect. Gellhorn (1958) suggested that the afferent nerve activities, including movements within facial muscles, determine the course of action within the hypothalamus (40). He indicated that muscle relaxants mediate hypothalamus activity in non-human subjects. However, he failed to explain the relationship between specific facial muscles and specific emotional experiences. Another hypothesis that might explain the role of facial feedback in the experience of one's emotions was introduced by Zajonc and Markus (41). This hypothesis indicates that affective experiences can be caused by the brain's regional and overall temperature, which is moderated by the air that goes through the nasal sinuses. According to this hypothesis, facial muscles can create negative emotions by altering the brain's temperature (5). Furthermore, this idea that decreasing negative emotional experiences alleviates the severity of depressive symptoms is coordinated with the theoretical framework of depression (42). According to this theory, depressed individuals have depressive belief systems based on negative assessments of factors that cause stress and harm them. In this model, negative thoughts, feelings, and behaviors are considered the secondary symptoms of depression that can impact the primary negative cognitive assessment model. That is to say that decreasing negative emotions in depressed individuals can, on the one hand, decrease negative and depressing thoughts and, on the other hand, indirectly impact the central cognitive assessment system.
Finally, based on a specific theoretical framework and previous studies, this study attempted to examine the effectiveness of intervention by electrical stimulation of facial muscles as a simple and cost-friendly treatment method to control the severity of depressive symptoms. While there are other methods available, including pharmacotherapy, psychotherapy, trans-cranial direct current stimulation (tDCS), and repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression, because of the complex nature and multifactorial etiology of this disorder, it is necessary to introduce new methods of treatment that are less expensive and impose a less financial burden on the health care system. In general, the findings of this study at a primary stage indicate that using functional electrical stimulation in some facial muscles can alter the type and severity of the emotional experience. Furthermore, although previous studies have investigated the effects of facial muscle electrical stimulation on mood enhancement, none have examined the role of frontalis muscle electrical stimulation in alleviating depressive symptoms. Therefore, the findings of this study are novel and demonstrate the efficacy of electrical stimulation of the frontalis muscles in reducing negative mood and improving depression compared to other studies in the same field.
Our study has limitations; thus, we have some suggestions for future research. First, different facial muscles are responsible for different emotional expressions of muscle, and it is possible that targeting certain muscles may be more effective than others in alleviating depressive symptoms. This study was limited in scope, as it did not examine the effects of electrical stimulation on all muscles involved in changes related to depression, including the orbicularis oculi muscle. We suggest conducting additional studies involving the electrical stimulation of more facial muscles and investigating their potential interaction. This could lead to more precise findings. Second, this study was limited in using EEG or MRI tools to investigate potential neuronal changes in participants related to depression and the effect of electrical stimulation on brain activity. Further research incorporating these techniques could help shed light on the underlying neural mechanisms involved in facial electrical stimulation and aid in developing targeted and effective treatments for depression. Third, although single-case experimental designs have their benefits in clinical research for examining individual progress and are applicable for feasibility studies, it is typically recommended to use group comparison designs like Randomized Controlled Trials (RCTs) as the preferred method (known as the "gold standard") for conducting such research because of their controlled research design. The limited number of participants, lack of a control group, and potential for the placebo effect in the current study necessitate further research with a controlled design to accurately assess the effectiveness of this intervention for treating depression. Future studies should aim to investigate the efficacy of facial electrical stimulation in a controlled experimental setting to obtain more precise and reliable results.
5.1. Conclusions
The findings of this study indicate that the functional electrical stimulation of facial muscles can be an effective tool in alleviating the severity of depressive symptoms. This method can successfully treat one's depression by reducing negative affect. While evidence supports its therapeutic benefits, including this research, more research is needed to fully understand the potential of this approach. This is especially important given the complex nature of depression and the various factors that can influence its development and progression. Factors such as the specific muscles stimulated, the intensity and duration of stimulation, and the underlying neural mechanisms involved must be carefully considered in future studies. Further exploration of this research area can lead to more accurate and efficient knowledge of new and interdisciplinary approaches to treating mood disorders, especially depression.