1. Background
Major Depressive Disorder (MDD) has turned into a significant health concern worldwide, as it affects 2 - 21% of the global population. According to the Global Burden of Disease study, depression was the fourth leading cause of disability within the previous decade and is assumed to become the second one in the 2020s (1).
However, depressive disorders have a wide range of classifications, categories, and attributes; sadness, loss, anger, and disinterest are the major persistent feelings of the affected individuals (2). Approximately one-third of the patients are resistant to the usual conventional pharmacological, psychological, and somatic therapeutic approaches known as Treatment-resistant Depression (TRD) (3). Irresponsiveness to the routine anti-depression strategies puts TRD individuals at increased risk for alcoholism, drug abuse, recurrent hospitalizations, and suicide (4).
To date, numerous efforts have been made to control depression in TRD patients, among which Electroconvulsive Therapy (ECT) and Transcranial Magnetic Stimulation (TMS) have gained the most attention. Despite the abundant administration of these interventions, even if they may lead to promising outcomes, it takes about 2 - 6 weeks for the individuals to relieve themselves from depression or suicidal ideation. Given that, researchers are probing for new strategies that might efficiently bridge the existing gap between the current approaches (3, 5, 6).
Ketamine is a glutamate receptor-blocking agent widely applied as an anesthetic agent in clinical practice; however, it has become a target of research for its potential antidepressant and anti-suicidal effects. The previous investigations showed that the antidepressant effect of ketamine initiates within 24 hours after application and gradually disappears by the end of a week (7, 8). Since 2019, the Food and Drug Association has approved the nasal form of ketamine, known as esketamine, to be applied for TRD under the supervision of healthcare providers (9). However, further investigations on the other forms of ketamine, including intravenous and oral forms, are limited (9, 10). Although the other forms of ketamine have not been officially approved for TRD, some studies have recommended several repeated infusions rather than a single dose to increase its efficacy and durability (11, 12). Although the theory about ketamine use for TRDs is not novel, the beneficial dosage and route of administration to achieve more effective outcomes remain a question (13, 14).
2. Objectives
The current study aimed to compare the effect of 1 and 2 mg/kg of oral ketamine in TRD subjects.
3. Methods
3.1. Study Population
The current Randomized Clinical Trial (RCT) was conducted on 29 patients suffering from TRD referred to the outpatient psychiatry clinics affiliated with Isfahan University of Medical Sciences from May 2019 to August 2021.
The research followed the tenets of the Declaration of Helsinki. The Ethics Committee of Isfahan University of Medical Sciences approved this study. The institutional ethics committee at Isfahan University of Medical Sciences approved all study protocols (IR.MUI.MED.REC.1398.632). The study was proposed to the Iranian Registry of Clinical Trials and accepted by the code number IRCT20090801002266N14. The study protocol was completely explained to the patients, and they were reassured regarding the confidentiality of their personal information. They also signed written consent to participate in the study.
Eighteen-to-sixty-year-old patients with a documented diagnosis of TRD using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria were included (15). Treatment-resistant depression was defined as a lack of response to at least two anti-depressive monotherapies of adequate dose and duration (4 - 6 weeks), including the current episode (15). The presence of any concurrent psychiatric disorders (psychosis, mania, or hypomania), recent history of drug abuse within the previous 3 months, pregnancy or lactation, untreated/uncontrolled hypo-/hyperthyroidism, the presence of any uncontrolled medical conditions, and the concurrent use of the agents interfering with ketamine use were defined as the exclusion criteria.
The patients entered the study by stratified permuted block randomization. Accordingly, all the subjects who met the criteria were included until the expected number of cases. The patients were randomly divided into two groups of interventions using Random Allocation Software. Accordingly, each patient was provided with a number, and they were allocated to one of the intervention groups.
The study was conducted in a double-blinded manner, as the patients and the psychiatrist who interviewed and followed the patients were blinded to the applied regimens.
3.2. Interventions
The first group of patients was treated with 2 mg/kg ketamine (Sterop, Belgium) applied twice a week for six weeks, while the second group administered 1 mg/kg ketamine (Sterop, Belgium) in a similar pattern.
The applied drugs were the injectable forms dissolved in fruit juice and given to the patients.
3.3. Outcomes
The primary outcome of this study was to assess the severity of depression and response to the treatment according to the questionnaires, including the Hamilton Depression Rating Score (HDRS) and Beck Depression Inventory-II (BDI-II).
The questionnaires were filled out before the interventions, every two weeks during the interventions, and within a week, a month, and two months after the end of the interventions. All the questionnaires were filled by or under the supervision of the psychiatry assistant who was responsible for conducting the study.
The secondary outcome of the study was to investigate the potential adverse effects related to oral ketamine according to the pharmacological references (16). The assessed adverse effects included headache, dizziness, elevated blood pressure, blurred vision, dissociation, nausea, drowsiness, lightheadedness or loss of consciousness, anxiety, and tachycardia.
3.4. Instruments
3.4.1. Hamilton Depression Rating Scale
The Hamilton Depression Rating Scale (HDRS) is a rating scale assessing the severity of depression. It consists of 17 items asking about the symptoms, including low mood, suicidality, irritability, tension, loss of appetite, loss of interest, and somatic symptoms. The questions are responded to on 3-, 4- or 5-point Likert scales. The higher scores correspond to more severe depression symptoms (17). The Persian version of HRDS has been validated by Shabani et al., who achieved Cronbach's alpha of 0.81 (18).
3.4.2. Beck Depression Inventory-II
The Beck Depression Inventory-II (BDI-II) is one of the widely used questionnaires to assess symptoms and the severity of depression. The BDI-II is a self-report measure containing 21 items, rating from 0 - 3. The lower scores represent milder depressive disorder. The Persian version of BDI-II was validated by Cronbach's alpha of 0.87 and test-retest reliability of 0.74 in 2005 (19).
3.5. Statistical Analysis
The obtained data were entered into the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 23. Descriptive data were presented as mean, standard deviation, percentages, and absolute numbers. Chi-square or Fisher's exact tests were applied to compare the categorical variables. Independent t-test was utilized to compare the continuous data. The trend of changes in response to the questionnaires was evaluated by repeated-measures ANOVA test. A P-value of less than 0.05 was considered significant.
4. Results
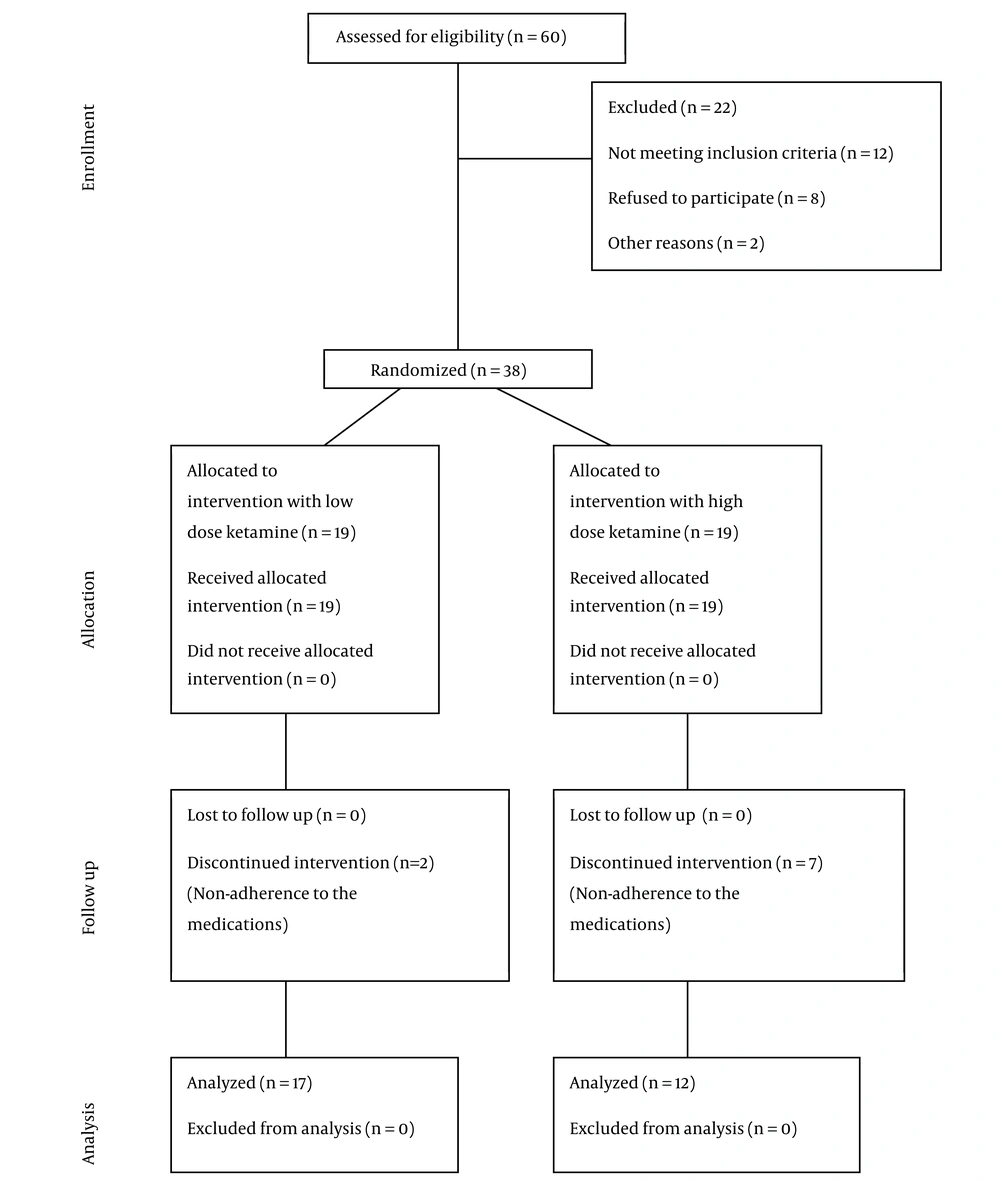
In the current study, data from 60 patients were gathered; among them, 22 patients were not included due to the exclusion criteria. The remaining 38 patients were randomly assigned into two equal treatment groups with oral ketamine of 1 mg/kg versus 2 mg/kg (low-dose versus high-dose). However, 2 patients in the low-dose ketamine-treatment group and 7 in the other group withdrew the therapeutic approach. Eventually, the analysis was performed on 17 and 12 patients treated with 1 mg/kg and 2 mg/kg ketamine, respectively. Figure 1 depicts the CONSORT diagram of the studied population.
The mean age of the participants was 37.29 ± 8.55, among whom 68.96% were females. The two groups were similar in terms of age (P-value = 0.64) and gender distribution (P-value = 0.60). Detailed demographic information is demonstrated in Table 1.
Baseline HRDS did not differ between the groups (P-value = 0.393). Further evaluations showed statistically significant efficacy of high-dose ketamine in the responses to HDRS (P-value = 0.0109); however, insignificant alterations were noted among the patients treated with low-dose ketamine (P-value = 0.630). The 2 intervention strategies did not show a significant difference (P-value = 0.975) (Table 2).
| Groups | Baseline | Intervention Time | Follow-up Time | P1 | P2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 6 Weeks | 1 Week | 1 Month | 2 Months | ||||
| Low-dose ketamine | 17.76 ± 7.50 | 16.17 ± 6.65 | 16.64 ± 7.85 | 17.64 ± 9.63 | 18.82 ± 10.60 | 17.17 ± 8.57 | 17.23 ± 8.57 | 0.630 | 0.975 |
| High-dose ketamine | 19.91 ± 4.90 | 18 ± 4.89 | 15.58 ± 4.33 | 16.25 ± 4.95 | 16.41 ± 6.33 | 17.25 ± 6.18 | 17.50 ± 6.62 | 0.0109 | |
| P3 | 0.393 | 0.427 | 0.675 | 0.650 | 0.489 | 0.980 | 0.929 | ||
a P1: effect of time, P2: effect of intervention, P3: comparison of interventions at each period.
The assessments of BDI-II revealed significant improvement over time in patients treated with low-dose ketamine (P-value = 0.018), while the high-dose ketamine did not affect BDI-II outcomes (P-value = 0.104). Furthermore, the comparison of the 2 interventions revealed insignificant differences (P-value = 0.719) (Table 3).
| Groups | Baseline | Intervention Time | Follow-up | P1 | P2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 6 Weeks | 1 Week | 1 Month | 2 Months | ||||
| Low-dose ketamine | 57.82 ± 7.46 | 55 ± 4.88 | 53.68 ± 6.25 | 53.31 ± 4.81 | 49.37 ± 10.57 | 50.62 ± 11.52 | 52.93 ± 5.51 | 0.018 | 0.719 |
| High-dose ketamine | 57.42 ± 13.88 | 56.91 ± 7.57 | 56.25 ± 6.09 | 53.19 ± 7.16 | 46.58 ± 16.51 | 51.50 ± 11.78 | 56.25 ± 5.31 | 0.104 | |
| P3 | 0.919 | 0.506 | 0.372 | 0.947 | 0.496 | 0.845 | 0.185 | ||
a P1: The effect of time, P2: the effect of intervention, P3: the comparison of the interventions at each period
The comparison of the frequency of ketamine-related adverse effects revealed significant differences in terms of drowsiness (P-value = 0.021) and lightheadedness (P-value = 0.004) (Table 4).
| Headache | Elevated Blood Pressure | Dizziness | Dissociation | Nausea | Drowsiness | Anxiety | Tachycardia | Blurred Vision | Lightheadedness | |
|---|---|---|---|---|---|---|---|---|---|---|
| Low-dose ketamine | 0.41 ± 0.87 | 0.11 ± 0.33 | 5.23 ± 5.15 | 0.94 ± 2.90 | 2.058 ± 3.96 | 4.70 ± 5.05 | 0.58 ± 1.12 | 0.058 ± 0.254 | 0.11 ± 0.33 | 0.88 ± 2.91 |
| High-dose ketamine | 0.66 ± 2.30 | 0 ± 0 | 6.083 ± 5.55 | 0.58 ± 1.24 | 2 ± 2.93 | 10.16 ± 3.68 | 2 ± 3.81 | 0.25 ± 0.62 | 0.33 ± 1.15 | 5.91 ± 5.55 |
| P-value a | 0.55 | 0.61 | 0.64 | 0.94 | 0.74 | 0.021 | 0.67 | 0.61 | 0.91 | 0.004 |
a Mann-Whitney U test
5. Discussion
According to the results of the current study, low-dose ketamine led to significant improvement in the scores achieved by the self-reported scale of DBI-II, while the high-dose regimen was accompanied by superior results of HDRS, a questionnaire that is filled out by a psychiatrist, who is an expert in the assessment of depression. However, we achieved no further logic for the controversy of our outcomes. Further investigations revealed insignificant differences in regard to the adverse effects induced by low- versus high-dose ketamine, except for drowsiness and lightheadedness that occurred remarkably more among those treated with higher doses.
By the late 1990s, growing levels of preclinical evidence represented the potential antidepressant effect of ketamine due to its N-methyl-D-aspartate (NMDA) antagonizing effects (3). The result is the inhibition of inhibitory interneurons, which leads to boosted excitation by raising glutamate levels in the interneurons of both the prefrontal cortex and the hippocampus (20). Accordingly, the first study in this regard was conducted in 2000 in which 7 patients received 0.5 mg/kg intravenous ketamine versus placebo over 40 minutes for 2 days. They evaluated their patients 4 times in a period of 3 days and achieved significantly promising outcomes that ignited the first thoughts for further investigations (21). Zarate et al. conducted the next study on 18 TRD patients with a follow-up period of a week. They injected 0.5 mg/kg ketamine in two apart doses with a week interval. As shown, 71% of the patients presented an immediate significant response, and 35% of the subjects maintained the response for at least 1 week. Accordingly, they proposed a theory that the psychoactive nature of ketamine is responsible for its clinical effect rather than a true improvement of neurobiological pathways leading to depression (8). In the next step, ketamine was compared with a psychoactive agent, midazolam, to assess whether ketamine effects are due to its NMDA receptor antagonizing or mind-altering effect. The outcomes of this study led to the dramatic superiority of ketamine (7).
Nevertheless, efforts went on to apply ketamine in non-invasive forms, and by 2019, the intranasal form of this agent was approved to be used under the supervision of healthcare providers. In this regard, some studies have been conducted. Lapidus et al. intervened with their patients using 50 mg of intranasal racemic ketamine. In comparison with a saline solution, this led to a remarkable improvement in symptoms within 24 hours (22). Fedgchin et al. were the other group of researchers who evaluated esketamine in doses of 56 and 84 mg versus placebo twice weekly and presented response initiation within 24 hours and improvement in depression intensity after 28 days (23). Similarly, Popova et al. evaluated similar doses, concluding response initiation from the first 24 hours to 28 days, as well as disease remission in the 28-day reassessment (24). Moreover, a systematic review and meta-analysis by An et al. culminated in promising outcomes assessing intranasal ketamine use for TRD (9).
The positive outcomes of ketamine use for TRD and other psychiatric disorders, such as mood disorders, post-traumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD), have prompted researchers to explore 2 aspects of ketamine use in these conditions, including the use of this agent from oral route and the long-term administration of ketamine in order to elongate the efficacy of this medication (25, 26).
Accordingly, it has been raised that long-term ketamine use can potentially result in changes in neurotrophic factors, leading to increased long-term potentiation, thus increasing neuronal synaptoplasticity and improving baseline clinical depressive symptoms (20). These outcomes led to further investigations in regard to administering ketamine in diverse doses, oral routes, and longer use to achieve an appropriate alternative treatment for TRDs.
Shiroma et al. conducted a six-month follow-up study in which they assessed 6 times repeated doses of ketamine versus a single subanesthetic dose and presented significant improvement in TRD response to treatment (27). The other study tried to investigate rapid and long-term efficacy of 6 times 0.5 mg/kg intravenous ketamine that showed significant improvement within the first 4 hours in both depressive symptoms and suicidal thoughts; however, the response sustained following the subsequent infusions. The long-term outcomes were dependent on the primary response (12). Phillips et al. conducted another study in which a course of six open-label ketamine infusions was administered thrice weekly over 2 weeks. Non-respondents received maintenance therapy as 4 other infusions once a week. They eventually concluded that repeated ketamine infusions have cumulative and sustained antidepressant effects. Reductions in depressive symptoms were maintained among responders through once-weekly infusions (13).
The application of ketamine from the oral route has been noted in more recent investigations as it may provide a persistent response condition. Domany et al. conducted a study in which oral ketamine was administered at 1 mg/kg dose three times a week for 21 days and concluded that repeated oral ketamine produced rapid and persistent amelioration of depressive symptoms in outpatients with TRD and was well tolerated (28). The other study by Hartberg et al. represented promising long-term outcomes for the oral use of ketamine as it led to a 70% decrease in the period of hospital stay and a 65% reduction in hospitalization of TRD patients. They even recommended the oral route rather than numerous intramuscular or intravenous applications (29). We found no study comparing different dosages of ketamine or applying 2 mg/kg of oral ketamine.
Despite all the efforts made to assess the efficacy of ketamine for TRD management, its potential adverse effects remained an unanswered question. Besides, despite the well-tolerability of ketamine in different reports, lightheadedness, dizziness, tiredness, headache, nervous floating feeling, and bad dreams are the most significant complications limiting its use (30-32). In agreement, the only point that lent our tendency to use low-dose ketamine is higher frequencies of adverse effects in high-dose treated cases.
5.1. Limitations
The most significant limitation of this study is the lose considerable number of participants due to withdrawal from the interventions.
5.2. Conclusions
According to the findings of this study, we achieved no conclusive superiority or efficacy of 1 mg/kg over 2 mg/kg of oral ketamine; however, considering the adverse effects, 1 mg/kg is preferred.