1. Context
Neurofeedback (NFB) training or electroencephalography (EEG) biofeedback training is categorized as a low-risk approach that makes the function of cognitive behavior enhanced by impacting the brain or, to be more exact, by adjusting its electrical activities. The relation between obvious cognitive functions and specific EEG-observed brain activity is well identified (1-3). Moreover, the effectiveness of NFB in improving performance in healthy individuals has been approved (4-6). Particularly, its great positive influence on the performance and competency of cognition has been confirmed by several studies (7-9), and it develops some sorts of memory (10-13). Applying sensorimotor rhythm (SMR) showed broad enhancement in attention (14, 15). The improvement of sleeping, declarative learning, and memory by SMR has been shown by other studies (3, 16). In addition, optimal performance among athletes, irrespective of the sports field (17), and emotion regulation are other aspects that could be enhanced (18-20). Akin to other effective medical treatments, the occurrence of side effects is quite probable and has been observed as a result of NFB (21).
Other authors (5, 22) claimed that relying upon the NFB protocol acts as a double-edged sword; it might either increase or decrease the symptoms of attention-deficit disorder (ADD)/attention-deficit hyperactivity disorder (ADHD). The applied design for conducting this study was A-B-A reversal; this design was for indicating that when theta (4 - 7 Hz) is inhibited and SMR (12 - 15 Hz) is increased, it improves symptoms. Quite the opposite, when the level of that declined, the symptoms of previous authors (23) proposed that it is the NFB protocol that determines whether uncontrolled epilepsy gets better or deteriorates. By having the theta decreased and an intensified SMR, the paroxysmal activity of a sleep EEG will be reduced by 18%; when it is reversed, the NFB protocol showed 29% progress in the epileptiform activity (13, 24, 25).
Some authors (21) listed individual reports on non-intended consequences from those who were practicing and receiving NFB that were posted on seven Internet web pages. Emotional side effects, namely agitation, anxiety, and liability, and psychological side effects, including enuresis, ticks, and muscle twitches, were announced (16, 21). Nonetheless, research was unable to measure the extent of the different effects and their length. Different side effects could be either short-term or long-term, some of which could be in between (e.g., headaches, fatigue, and irritability) or severe (e.g., epilepsy, emotional dysregulation, manic episodes, (21, 24), and memory problems (26-28)). Many of those moderate side effects vanish after a short time after the end of the training session (15, 29). Based on well-controlled trials, various causes of side effects have been described. The primary causes are applying an inappropriate NFB protocol (21, 22, 23) together with the number of sessions ignoring the client’s conditions. What is more, whenever a general protocol is conducted irrespective of different mental statuses of individuals who have distinct prognoses (e.g., comorbidities) and different EEG activity (21, 30), side effects are inevitable.
In crude terms, power-increasing protocols are more attributed to causing side effects rather than power-decreasing ones (9, 21, 31). In the case of having training sessions extended more than standard, side effects could also occur (32, 33). Although the side effects have been declared, no systematic study showing the probability of side effects was accessible. Attention-deficit hyperactivity disorder and epilepsy are disorders that have been the target of behavioral interventions; although all the disorders have been investigated, a great deal of attention has been given to them. Alleviating the signs and symptoms of impulsivity, hyperactivity, and lack of concentration is corroborated by deploying NFB (2, 24, 34, 35).
The abatement of epileptic symptoms during and after NFB has been supported by a sheer number of studies (36-38).
2. Objectives
One must not lose sight of the fact that clinical NFB training has proven to be quite beneficial; however, there has been less focus on its drawbacks. The systematic examination of the temporary side effects of regular NFB protocols and juxtaposition of them with a sham group via a double-blind study is the primary goal of this inquiry among patients suffering from ADHD and epilepsy.
3. Evidence Acquisition
According to instructions of MOOSE (meta-analyses of observational studies in epidemiology) and PRISMA (preferred reporting items for systematic reviews and meta-analyses protocols), the current study was conducted (39). The current review scrutinized the English and Persian electronic published studies within March 1975 and March 2022. To specify the correlated literature pertaining to ADHD and epilepsy protocols, a preliminary keyword search was implemented. Manifold electronic database was probed involving Scopus, Social Sciences Citation Index (SSCI), PubMed, Google Scholar, CABI: CAB Abstracts and Global Health, Web of Science, Current Contents Connect, and Inspect and Persian electronic databases (i.e., Magiran, SID, Iran Medex, and Irandoc).
An identical method of research and keyword combination was applied for both English and Persian databases. The all-inclusive keyword searching included these items: “randomized controlled trial (RCT)” OR “clinical trial” AND “neurofeedback” OR “slow cortical potentials” OR “EEG” OR “biofeedback” OR “theta-beta protocol” OR “SMR protocol (neurofeedback intervention)” AND “epilepsy” AND “ADHD”, OR “attention deficit hyperactivity disorders” OR “attention deficit hyperactivity disorder”.
4. Study Selection
Along with various databases, published reviews and reference lists of the acquired papers were searched. Furthermore, the authors benefited from the expert opinion of those who have not published their works (gray literature). Removing the repetitive essays, the rest were evaluated by two autonomous reviewers based on the titles and abstracts (S. H. and M. R.). The philosophy behind appointing clinical trials was to be assisted by a higher control and to appraise the rate of NFB effectiveness more exactly. The tests indicating cognitive-behavioral and neurological conditions, diagnosis according to the Diagnostic and Statistical Manual (DSM), and randomized sampling were considered other inclusion paradigms. The studies conducted on particular populations (e.g., brain injury and stroke), articles released before 1975, letters or editorial articles, and review articles without basic sources were excluded. The exclusion criteria were those papers being published earlier than 1975 and those articles, editorials, and reviews lacking original data. The exclusion criteria included studies focusing on specific groups, such as brain injury and stroke. In addition, publications related to a specific population were removed. However, the publication with the highest validity was chosen to be analyzed. Moreover, being peer-reviewed for all English and Persian papers played a key role in their selection.
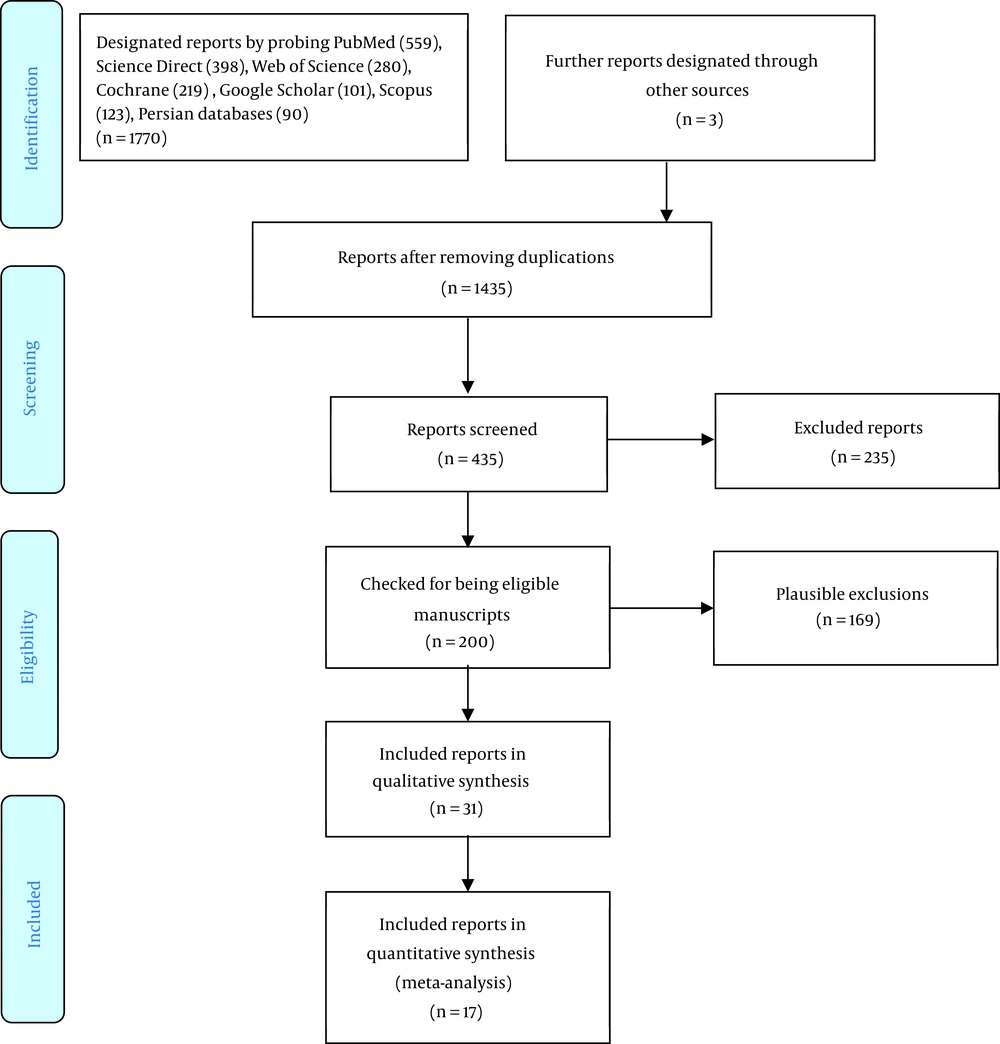
Participants either suffering from ADHD and epilepsy diagnosis or based on a broad-band rating scale measure were considered higher than the cutoff point, for example, the TOVA (Test Of Variables of Attention) and IVA (The Integrated Visual and Auditory) (40, 41). All age groups with ADHD and epilepsy participated in this study. Studies were randomized controlled trials (RCTs). All trials were included unrelated to intervention quality/characteristics. Regardless of the level of qualification or features of the trial, almost all the trials were counted. The sole exclusion criterion was having a comorbidity, such as autism. The analyzed studies were those from which the symptoms of ADHD and epilepsy were observed. The same happened when NFB was applied along with another treatment, as the result could not be purely attributed to NFB. Unrelated to the subject of the studies (e.g., type of pharmacotherapy), those who met the abovementioned standards were calculated in the final analysis unless there was an outcome related to ADHD and/or epilepsy. Figure 1 shows the results of the first search for papers and depicts how the final papers were culled to be examined in the current review. At every level of the process, there were two autonomous examiners who resolved inconsistencies by consultation with their colleagues. Nevertheless, there is no quorum for carrying out a meta-analysis to incorporate at least three advised by the literature (42-51).
5. Data Extraction
Review Manager software (version 5.3) was utilized to register and record the characteristics of all the data concerning the sample and the designs of the trials. The items that were important for extracting the data included the properties of the study, the publication year, the gender and qualities of the participants, the intervening and controlling method, outcomes, and principal results. One of the examiners was responsible for data extraction, and they were checked separately by another one. The chosen variables to be investigated were in accordance with a pragmatic evaluation of the study results (Figure 1).
5.1. Quality Assessment of Studies
To monitor the quality of the process, following the standards of the JBI (Joanna Briggs Institute) prevalence critical appraisal tool (12), all the original reports were reviewed by a couple of the researchers (Y. M. and H. R.). The quality level of the evaluation was fluctuating between 55% and 100%. The papers with a low score (< 60%, n = 3) were eliminated. For the analysis, only the scores above 60% were chosen, and the rest (n = 3) were discarded. Third-party opinion (S. H.) was exerted to settle the dispute between researchers over the quality controlling of the process. To guarantee that up-to-date publications were selected, investigations were performed repeatedly. The latest search was performed on April 8, 2022. The best-quality articles associated with the mentioned keywords were chosen. The search process was repeated many times to be assured of referring to the latest publications, which the final one was implemented on April 8, 2022. The best of the team was performed to not spare any publication containing the keywords (Table 1).
5.2. Statistical Analysis
The symptoms of epilepsy were analyzed discretely. For calculating its pooled prevalence and confidence intervals (CI) (95%), the model used was the random effect, utilizing the mean command in Stata software (version 14). The rate of 50% and above in I2 statistics among studies was the criterion for heterogeneity; however, it ranged from 0 to 100 (52). The calculation of pooled measure and 95% CI was assessed by a forest plot (53). For heterogeneity, the used program was meta-regression analyses, and the year of publication, average age, gender, and follow-up duration were examined by univariate meta-regression (UMR). A P-value < 0.05 by Stata software (version 14) was regarded as the significant level for the entire process. By the graphical method (each trial was juxtaposed to the standard error deploying a funnel plot) and statistical tests (Egger’s test and Begg’s test), the effect size of the publication bias was measured.
6. Results
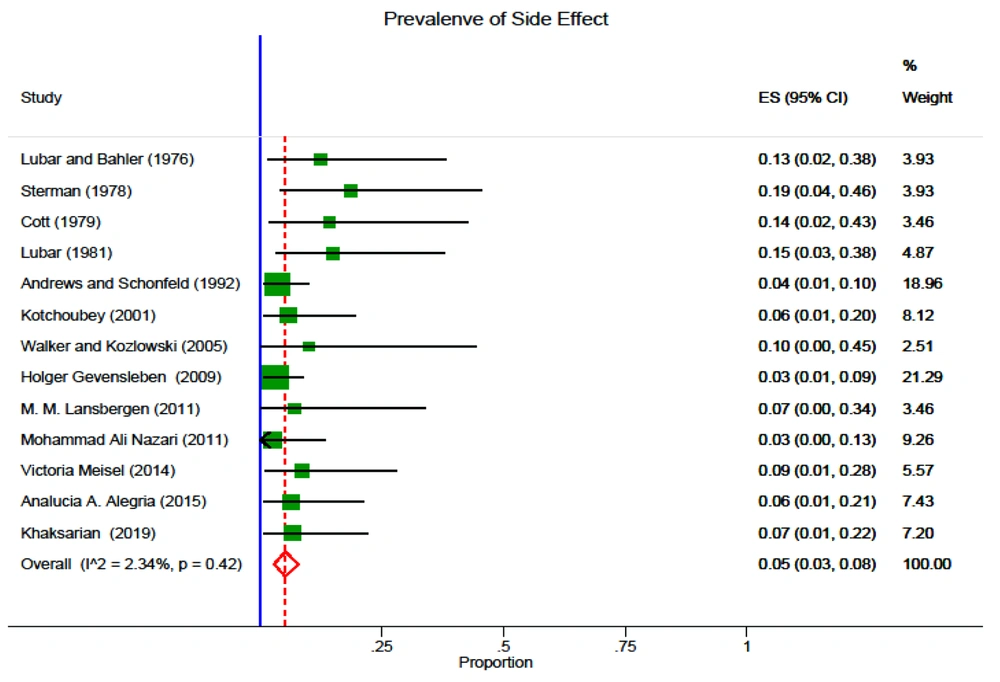
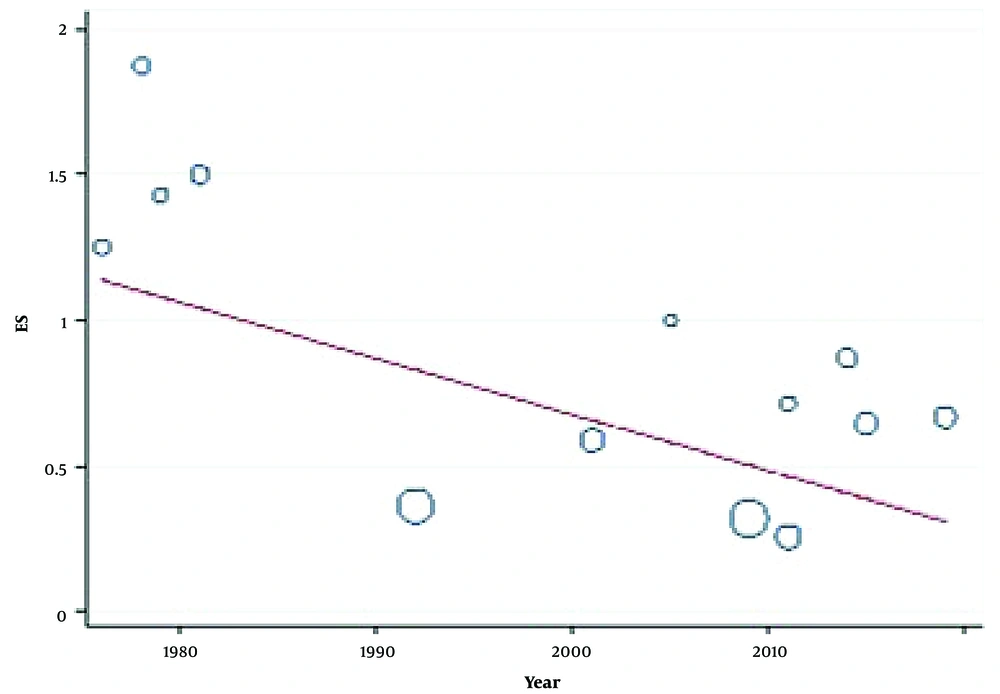
Random effect was the model deployed to measure the CI of 95% and pooled prevalence of side effects, which was reckoned up as 0.05 (95% CI: 0.03 - 0.08). Based on I2 statistics, the Galbraith diagram, and evaluating heterogeneity, its rate was 2.34%; nevertheless, the Galbraith diagram did not indicate any heterogeneity. Univariate meta-regression was the model for detecting the occurrence rate of the side effects in the year of the publication. In spite of the downward tendency of the trend, the results were not statistically significant (Figure 2).
| ID | First Author | Disorder | Duration of Treatment | Simultaneous Stimulant Medication | NFB Protocol | Control Condition | T - C | Age Range (y) | Side Effect (M) | Side Effect (SD) | Side Effect (N) | Type of Side Effect | Gender |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lévesque et al. (2006) (5) | ADHD | 10 - 12 weeks | NA | Theta/beta training | Waitlist | 15 - 5 | 8 - 12 | Not reported | - | - | - | Male and female |
| 2 | Gevensleben et al. (2009) (43) | ADHD | May 2005 to December 2007 | NA | Theta/beta training/(SCP) | Waitlist | 59 - 35 | 8 - 12 | 11.3 | (5,9) | (3) | Dizziness | Male and female |
| 3 | Lansbergen et al. (2011) (54) | ADHD | 4 months with 2 sessions per week, in total 30 sessions | NA | Theta suppression/SMR | Waitlist | 8 - 6 | 8 - 15 | 9.3 | (4,7) | (1) | Headache | Male and female |
| 4 | Liechti et al. (2014) (10) | ADHD | 12 weeks | MPH | Theta/beta training | Waitlist | 13 - 5 | 8.5 - 13 | None | - | - | - | Male and female |
| 5 | Maurizi et al. (2014) (4) | ADHD | 12 weeks | NA | Theta/beta training | Waitlist | 25 - 10 | 8.5 - 13 | Not reported | - | - | - | Male and female |
| 6 | Meisel et al. (2014) (7) | ADHD | 40 weeks | NA | Theta-beta training | MPH | 11 - 12 | 7 - 14 | 6.3 | (3,4) | (2) | Dizziness | Male and female |
| 7 | Mohammadi et al. (2015) (44) | ADHD | 15 sessions/every session 45 minutes | MPH | SMR/theta-beta training | Waitlist | 16 – 16 | 9 - 15 | None | - | - | - | Male and female |
| 8 | Geladé et al. (2016) (55) | ADHD | 10 - 12 weeks | MPH | Theta/beta training | Waitlist | 112 - 110 | 7 - 13 | None | - | - | - | Male and female |
| 9 | Geladé (2017) et al. (56) | ADHD | 6 months | MPH | Theta/beta training | Waitlist | 112 - 110 | 8 - 13 | None | - | - | - | Male and female |
| 10 | Strehl et al. (2017) (45) | ADHD | 3 months | NA | Theta/beta | Waitlist | 75 - 69 | 7 - 9 | Not reported | - | - | - | Male and female |
| 11 | Shereena et al. (2018) (46) | ADHD | 3.5 - 5 months | NA | Theta/beta training | Waitlist | 15 - 15 | 6 - 12 | None | - | - | Male and female | |
| 12 | Rubia et al. (2019) (47) | ADHD | 2 weeks | MPH, dexamphetamine | fMRI | Waitlist | 18 - 13 | 12 - 17 | None | - | - | - | Male |
| 13 | Minder et al. (2018) (48) | ADHD | 10 - 14 weeks | NA | Theta-beta training | Waitlist | 47 - 30 | 8.5 - 16 | None | - | - | - | Male and female |
| 14 | Nazari et al. (2011) (49) | ADHD | 10 - 12 weeks | NA | Theta-beta training | Waitlist | 26 - 13 | 8 - 12 | 8.1 | (4,17) | (1) | Headache | Male and female |
| 15 | Bakhshayesh et al. (2011) (50) | ADHD | 30 sessions | NA | Theta-beta training | Waitlist | 18 - 17 | 6 - 14 | Not reported | - | - | - | Male and female |
| 16 | Alegria et al. (2015) (51) | ADHD | 2 weeks | NA | fMRI | Waitlist | 18 - 13 | 12 - 17 | 15.11 | (6,74) | (2) | Dizziness | Male |
| 17 | Aggensteiner et al. (2018) (14) | ADHD | 25 sessions | NA | SCP | Waitlist | 144 | 7 - 9 | Not reported | - | - | - | Male and female |
Abbreviations: T, treatment group; C, control group; M, mean; SD, standard deviation; N, number; NFB, neurofeedback; NA, no action; MPH, methylphenidate; SCP, slow cortical potentials; SMR, sensorimotor rhythm; fMRI, functional magnetic resonance imaging.
6.1. Meta-regression
Univariate meta-regression was the program used with the aim of pinpointing heterogeneity. The items of year of publication, average age, gender, and follow-up period were listed independently, and the method of approximation was pursuant to the restricted maximum likelihood. The meta-regression suggests that the primary root is the year of publication by which heterogeneity is impacted. The year of publication and side effects of NFB were associated positively (Figure 3). The association between the year in which the papers were published and the side effects was positive (Figure 3).
6.2. Assessment of Publication Bias
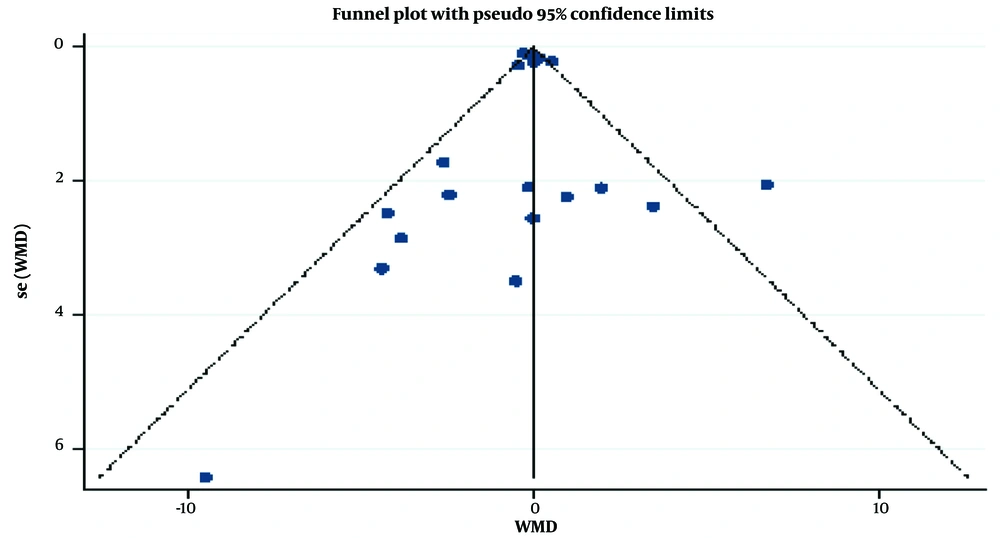
The symmetrical trend of the funnel plot confirms the non-existence of any publication bias, as implied by Egger’s test (P = 0.98) and Begg’s test (P = 0.23) (Figure 4).
7. Discussion
Homing in on scrutinizing how NFB affects the patients suffering from ADHD and epilepsy, the authors commenced this meta-analysis, which is the first paper covering this issue with this population. Being juxtaposed with the control groups of ADHD and epilepsy, NFB disclosed no problematic side effects, according to this meta-analysis. Moreover, NFB has been affirmed by preceding reviews as an effective treatment for almost all children and adolescents (34, 35). Nonetheless, the commonplace treatments for ADHD and epilepsy are still medical and family-based interventions. Along with the confirmed effects of these two interventions, a notable rate of individuals suffering from ADHD and epilepsy have benefitted neither from medicine nor from psychological interventions, according to their reports. Although EEG NFB is still a well-known neurological treatment that lacks the regional resolution of functional magnetic resonance imaging (fMRI) for regulating the brain waves in profound regions, it has some advantages, such as being economical and assessing temporal regions.
Although EEG-based NFB is outperformed by fMRI when it comes to spatial resolution and recording the waves of central regions of the brain, it has remained the most prevalent treatment owing to its temporal resolution and being economical (68-70). The results are not aligned with the studies that are against using medical treatments for ADHD and epilepsy patients. However, it is apparent that in NFB, there is always the probability of making mistakes in interpreting the patients’ assessments, such as misunderstandings, over-expectations, and irrelated side effects. This study established a strong base to advocate the equal positive efficacy of various protocols in experimental groups of ADHD and epilepsy. Children and teenagers, understandably, are interested in this treatment as it is aided by animation grounded on operant conditioning. Therefore, it could be particularly developed for them.
Practicing identical protocols in a majority of the clinical trials indicates the presence of no heterogeneity. Yet, a meticulous exploration among studies revealed that the acquired data were reliable only when standard protocols were involved, which, in turn, are promising for proceeding with treatment with these protocols. Eventually, the lack of any information related to the percentage of participating patients was another contributing factor in raising the heterogeneity. It was even fortified on account of including diverse studies, such as fMRI and EEG.
Additionally, another problem that ensued from using different modalities was targeting distinct areas of the brain. Electroencephalography feedback was the most frequently used one among them; therefore, it plays a key role in determining the variance of the resulting data. It should be considered that the characteristics of the sample and length of the treatment had no influence on the submitted effect size. The scope of this review was restricted by a number of elements that should be observed while interpreting this meta-analysis.
To begin with, there is the probability of receiving two different treatments, including medicine and NFB, at the same time by which the patients’ reports will be impacted; therefore, the insight of the patients and tolerance of symptoms will affect the reports rather than solely the side effects. Having expressed these issues, the findings of this study confirm that patients might be assuaged by the influence of other factors that are not directly related to treatment per se. Along with it, the role of the year of publication and the sample size should not be ignored. The rate of subjects participating in the studies fluctuated from 3 to 37; however, in the majority of them, the participants consisted of less than 15 (71). As confirmed by strong evidence, among those who are suffering from obsessive-compulsive disorder (OCD), men are more exposed to contracting serious illnesses. The results also might vary according to the technology of NFB instruments; the ones obtained earlier might present different results from those benefitting from the new technology.
Moreover, to interpret the findings of this study, it is necessary to put emphasis on the focus of the preceding research on ADHD and epilepsy, most of which have been exploring the effectiveness of NFB in a special disorder. Consequently, the proliferation of studies on ADHD and epilepsy prompted the authors to perform a meta-analysis on these two areas. There might be some studies that have reported not desirable effects of NFB; nevertheless, they were excluded from the present meta-analysis due to not discussing question disorders. Additionally, as the studies regarding children and young adults attract the great attention of scientists and experts, the applied protocols are mostly standard and approved; therefore, akin to the previous experiments, the least possible extent of the side effects is anticipated. Nonetheless, the authors are oblivious to the number of included studies that examined the case and implemented the protocol according to the initial brain waves. The accuracy of analyses was an important criterion for being included in this meta-analysis; consequently, some of the analyses were removed due to lack of heterogeneity, which could change the results.
Regarding epilepsy, being a heterogeneous disorder is another issue that gives rise to various diagnoses with comorbid conditions. For this reason, it is quite probable that the included protocols are highly impacted by the severity and fluctuations of the disorder inasmuch as the effectiveness of NFB varies depending on the time of performance. It implies and, at the same time, justifies the lack of accuracy of the entered data. Nevertheless, the way that protocols were performed is disputable. Although most of the included studies shared the same protocol research, these same protocols could be carried out at various times and methods by different researchers. For the forthcoming research, it is suggested to evaluate other significant disorders whose probability of being cured by NFB is high. Moreover, applying heterogeneous protocols for the same disorder is advised to discern the differential aspects of each. It is also proposed to measure the effectiveness of NFB by using comprehensive, valid lists. Lastly, the data of the current meta-analysis resulted from the limited side effects of NFB, which might not be in accordance with some other analyses; therefore, conducting further meta-analyses is implied.
There are researchers who are free from assessment bias, as they believe that the patients’ reports might not purely reflect NFB, and other factors are included. Secondly, as there was no access to the information related to how the general functionality of the patients (e.g., preparedness for school, academic accomplishments, and social skills) was influenced by the symptoms, it was not possible to measure the effect of the interventions on them. Thirdly, there was no opportunity to calculate the function of the moderators, such as the intensity of the disorders, which produced some changes in the data. For this reason, it is required to carry out further studies to assess them.
7.1. Conclusions
Overall, the results of this meta-analysis support the studies using NFB as a safe method for treating neurological disorders. This treatment can show objective effectiveness with the help of precise application and the use of quantifiable measurements. Additionally, since many ADHD and epilepsy sufferers are children and teenagers, this intervention is considered a complementary and safe method.