1. Background
Schizophrenia is a chronic mental health disorder that impacts nearly every aspect of an individual's functioning, with a lifetime prevalence of approximately 7 per 1000 individuals in the general population (1). While schizophrenia is characterized by a variety of psychotic symptoms, research indicates that negative symptoms and cognitive impairments have a more profound impact on patient outcomes and quality of life (QOL) (2-4). Current antipsychotic medications are insufficient in addressing the persistent cognitive symptoms associated with the disorder (5).
Oxidative stress is hypothesized to play a neurobiological role in the onset of schizophrenia and the associated cognitive decline (6). It has been linked to cognitive impairments in psychiatric populations and is being investigated as a potential pathophysiological factor in schizophrenia (7, 8). The antioxidant glutathione (GSH) protects cells from the harmful effects of reactive oxygen species (ROS) by facilitating their transport and removal (9, 10). Reducing oxidative stress in patients with schizophrenia has been proposed as a beneficial adjunctive therapy. N-acetylcysteine (NAC), a precursor to GSH, is a promising candidate for such treatment (11-13).
2. Objectives
Despite the potential benefits, there is a notable gap in the literature regarding the efficacy of NAC in improving symptoms in schizophrenia patients. This study is innovative in its approach by measuring both positive and negative cognitive symptoms, as well as the peripheral blood levels of GSH, in schizophrenia patients receiving NAC. The objective of this study was to evaluate the effect of NAC as an adjunctive treatment for schizophrenia.
3. Methods
3.1. Study Design
This study is a double-blind, randomized, controlled clinical trial involving patients with schizophrenia. Participants were referred to the outpatient university-affiliated clinic of Khorshid Hospital in Isfahan, Iran, from January to October 2022. Randomization was performed using a random number generator program, ensuring that both patients and clinicians were blinded to the treatment assignments.
3.1.1. Inclusion Criteria
- Definite diagnosis of schizophrenia disorder based on DSM-V criteria
- Stable illness for months, not in the acute phase
- Age between 19 to 65
3.1.2. Unmet Criteria
- Substance use disorder
- Acute phase
- Co-existing of another psychological disorder (depression, bipolar disorder, …)
- History of severe physical illnesses
- Pregnancy or breastfeeding
- History of anti-platelets or anti-coagulant treatment
- History of chloroquine, insulin, or nitroglycerin treatment
- History of electroconvulsive treatment (ECT) in the past 6 months
3.1.3. Exclusion Criteria
Discontinuation of NAC was considered if complications such as dizziness, headache, nausea, vomiting, heart palpitations, cardiac arrhythmia, shortness of breath, dry mouth, blurred vision, skin itching, or weakness occurred. The sample size was determined to be 35 patients in each group, based on the formula provided (14).
3.2. Study Protocol
After enrollment, patients were randomly assigned in a double-blind manner to either the case or control group, ensuring that both patients and clinicians assessing the patients were blinded to group assignments. Individuals in the case group received 600 mg of NAC (Hakim Pharmaceutical Co, Tehran, Iran) once daily in addition to their routine treatment, which consisted of 300 to 1000 mg of chlorpromazine daily. Patients in the control group continued their routine treatment with chlorpromazine at the same dosage range, supplemented by a placebo. Before the initiation of treatment, and at 1 and 2 months after the conclusion of treatment, all patients were interviewed in a clinical setting regarding medication usage. They were also asked to complete the Scale for Assessment of Positive Symptoms (SAPS), the Scale for Assessment of Negative Symptoms (SANS), and the neuropsychiatry unit cognitive assessment tool (NUCOG). Additionally, the peripheral level of GSH was measured for each group before the intervention and after 2 months (Figure 1).
3.3. Tools
The SAPS, designed by Anderson, consists of 35 questions evaluating five groups of symptoms: Hallucinations, delusions, bizarre behavior, general thought disorder, and inappropriate affect. Each item is scored on a 6-point scale from 0, indicating the absence of symptoms, to 5, indicating the most severe condition of the symptoms. Higher scores reflect more severe symptoms. The SAPS is considered valid compared to similar tools and is used to measure treatment effectiveness in clinical research. The validity and reliability of the Persian version of this questionnaire have been confirmed (15).
The SANS includes 24 questions assessing negative symptoms in five areas: Affective flattening or blunting, alogia, avolition-apathy, anhedonia-asociality, and attention. The validity and reliability of the Persian version of this questionnaire have been confirmed and discussed elsewhere (16, 17).
The NUCOG evaluates cognitive function across five domains: Attention, visuospatial domain, memory, executive function, and language, with 21 questions. Each subscale is scored from 0 to 20, with a total score range from 0 to 100. The NUCOG offers face validity, an appropriate range (covering all cognitive aspects, including executive functioning), multidimensionality (providing separate scores for distinct cognitive domains and a cognitive profile), brevity (completion in less than 20 minutes), and ease of learning. The validity and reliability of the Persian version of this questionnaire have been confirmed (18).
This study was approved by the Isfahan University of Medical Science Ethics Committee (IR.ARI.MUI.REC.1401.013) and the Iranian Registry of Clinical Trials (IRCT20201119049445N2). Informed consent was obtained from all participants before the study commenced.
3.4. Statistical Analysis
The collected data were analyzed using SPSS version 20 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA). Variables were measured using mean ± standard deviation and relative frequency indicators. The Kolmogorov-Smirnov goodness-of-fit test was employed to assess the distribution of quantitative variables. Depending on whether the data followed a normal or non-normal distribution and whether the variables were quantitative or qualitative, appropriate statistical tests were applied. These included Fisher's exact test, Student's t-test, one-way ANOVA, Tukey's test, and Pearson correlation coefficient. A significance level of 0.05 was considered for all statistical tests.
4. Results
After sampling, 35 patients were enrolled in each treatment group. However, five patients from the control group and six from the NAC group dropped out of the trial before the first month. Two patients withdrew due to the recurrence of an acute phase of schizophrenia, while others left the study due to either withdrawal of consent or substance dependence. Demographic information of the study population is presented in Table 1. The outcome variables in both groups followed a normal distribution (P > 0.05). The results were analyzed using the ANCOVA repeated measures test.
| Variables | Placebo (n = 35) | Treatment (n = 35) | P-Value |
|---|---|---|---|
| Age (y) | 37.5 ± 11.5 | 33.9 ± 7.9 | 0.128 |
| Gender | 0.231 | ||
| Female | 21 (60) | 16 (45.7) | |
| Male | 14 (40) | 19 (54.3) | |
| Duration (y) | 14.2 ± 9.4 | 12.5 ± 6.5 | 0.370 |
| Education | 0.443 | ||
| Before college | 11 (31.4) | 8 (22.9) | |
| College | 14 (40.0) | 12 (34.2) | |
| After college | 10 (28.6) | 15 (42.9) | |
| Complications b | 0.205 | ||
| Yes | 31 (88.6) | 27 (77.1) | |
| No | 4 (11.4) | 8 (22.9) |
Demographic Information of the Study Population a
4.1. Evaluation of Crude P-Value
At this stage, the outcome variables in the two study groups were compared without adjusting for other variables, such as age, gender, education, disease duration, and treatment follow-up times. As shown in Table 2, there was no significant difference between the primary group and any of the studied variables. However, significant differences were observed in the levels of GSH at the end of the second month, the SANS at the end of the first month, the visuoconstructional variable at the end of the second month, and the total score of the NUCOG at the end of the second month.
| Variables and Months | Placebo (n = 35) | Treatment (n = 35) | Crude P-Value | Adjusted Effect Size P-Value b | Follow-up Effect P-Value | Modifier Effect P-Value |
|---|---|---|---|---|---|---|
| GSH | 0.004 c | - | - | |||
| Base | 2.17 ± 0.71 | 2.23 ± 0.69 | 0.709 | |||
| 2 | 1.98 ± 0.66 | 2.32 ± 0.59 | 0.044 | |||
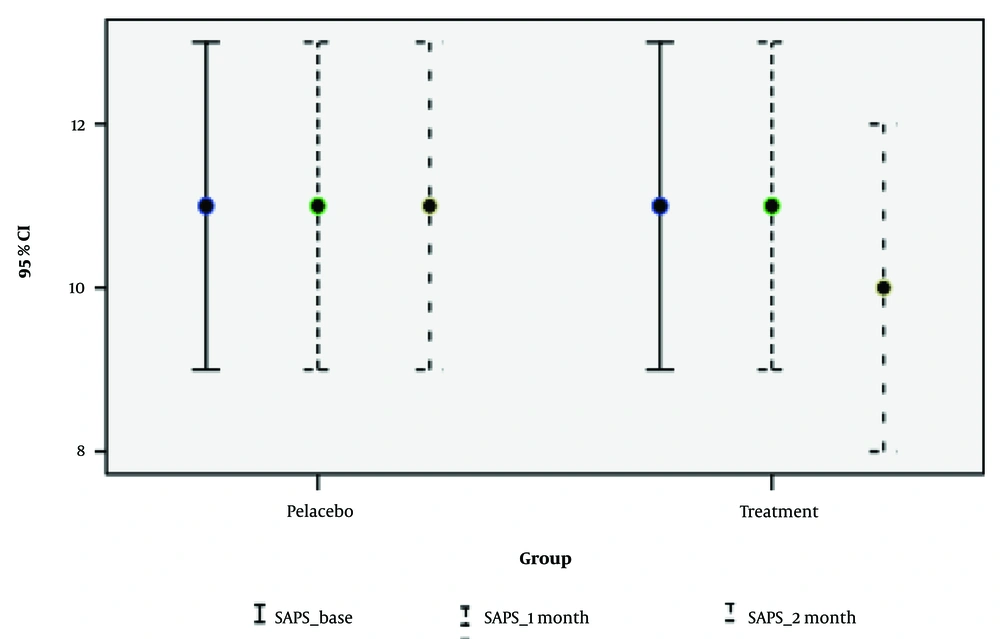
| SAPS | 0.004 c | 0.573 | 0.158 | |||
| Base | 11.17 ± 5.73 | 10.97 ± 5.37 | 0.881 | |||
| 1 | 11.61 ± 5.81 | 10.65 ± 5.55 | 0.492 | |||
| 2 | 11.50 ± 5.88 | 10.62 ± 5.21 | 0.881 | |||
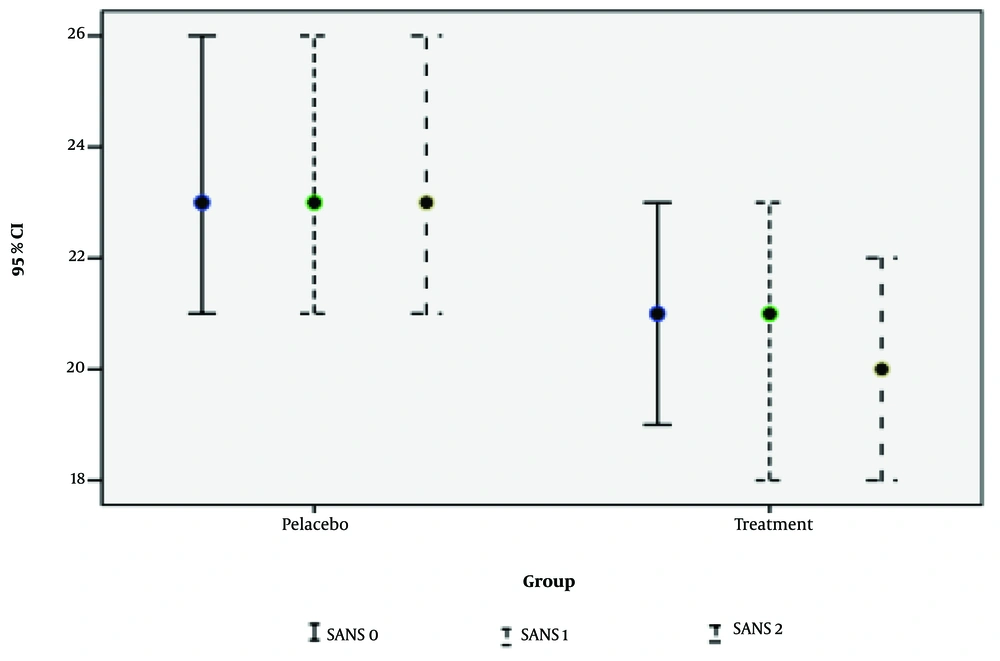
| SANS | 0.062 | 0.117 | 0.130 | |||
| Base | 23.83 ± 7.21 | 20.83 ± 5.98 | 0.062 | |||
| 1 | 24.18 ± 7.26 | 20.91 ± 5 .97 | 0.048 | |||
| 2 | 23.80 ± 7.10 | 20.72 ± 5.74 | 0.073 | |||
| Attention | 0.545 | 0.590 | 0.545 | |||
| Base | 11.51 ± 2.02 | 11.80 ± 1.59 | 0.513 | |||
| 1 | 11.61 ± 2.04 | 11.82 ± 1.60 | 0.629 | |||
| 2 | 11.83 ± 1.82 | 12.03 ± 1.80 | 0.671 | |||
| Memory | 0.971 | 0.259 | 0.434 | |||
| Base | 12.17 ± 2.36 | 12.34 ± 1.73 | 0.730 | |||
| 1 | 12.12 ± 2.41 | 12.35 ± 1.76 | 0.653 | |||
| 2 | 12.27 ± 2.45 | 12.59 ± 1.84 | 0.574 | |||
| Executive | 0.216 | 0.589 | 0.662 | |||
| Base | 11.16 ± 2.57 | 11.27 ± 2.91 | 0.862 | |||
| 1 | 10.97 ± 2.54 | 11.37 ± 2.99 | 0.560 | |||
| 2 | 10.92 ± 2.90 | 11.64 ± 3.11 | 0.361 | |||
| Visual-constructional | 0.003 c | 0.074 | 0.035 c | |||
| Base | 11.31 ± 1.71 | 11.57 ± 1.85 | 0.548 | |||
| 1 | 11.09 ± 1.84 | 11.65 ± 1.92 | 0.231 | |||
| 2 | 11.13 ± 2.10 | 12.21 ± 1.78 | 0.039 | |||
| Language | 0.997 | 0.263 | 0.997 | |||
| Base | 16.09 ± 1.56 | 16.09 ± 1.48 | 1.000 | |||
| 1 | 16.03 ± 1.59 | 16.09 ± 1.51 | 0.879 | |||
| 2 | 16.03 ± 1.59 | 16.14 ± 1.60 | 0.802 | |||
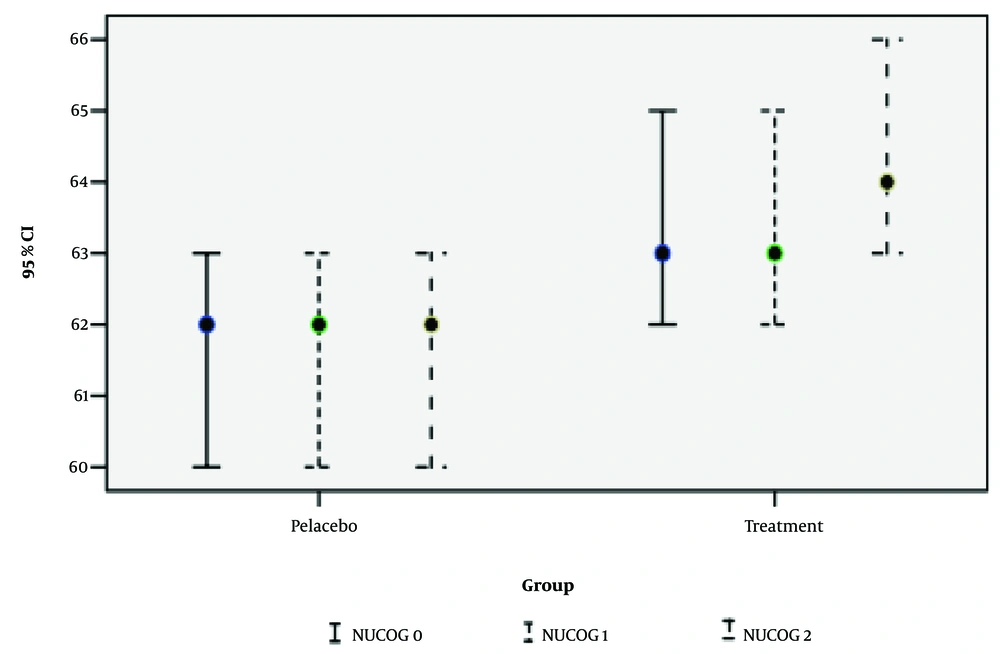
| NUCOG | 0.008 c | 0.870 | 0.046 c | |||
| Base | 62.24 ± 4.07 | 63.07 ± 3.80 | 0.382 | |||
| 1 | 61.82 ± 4.16 | 63.28 ± 4.01 | 0.148 | |||
| 2 | 62.18 ± 4.24 | 64.60 ± 4.10 | 0.030 |
The Effect of Add-on N-Acetylcysteine with Rout-in Therapy on Outcomes at Baseline, One, and Two Months Follow-up on Inpatients of Schizophrenia a
4.2. Evaluation of Adjusted Effect Size P-Value
Crude P-value results are not statistically reliable because they do not account for differences introduced by variables such as demographic factors. The adjusted effect size P-value, which controls for baseline values of age, gender, disease duration, and education in the statistical model, provides higher statistical reliability. As seen in Table 2, significant differences were found in the GSH, SAPS, visuospatial, and NUCOG variables between the treatment and placebo groups by the end of the study period.
4.3. Assessment of Follow-up Effect P-Value
The follow-up effect was examined to determine whether the continuation of drug use into the second month had a significant impact on the outcome variables. The P-value for this index was not significant for any of the variables, indicating no significant difference in drug effect between the first and second months.
4.4. Analysis of Modifier Effect P-Value
In this study, we examined whether the changes observed from the first month to the second month in the treatment group were significant when compared to the placebo group. The follow-up analysis focused on the treatment group, specifically comparing changes between the first and second months. To assess the modifier effect, we evaluated the changes from the first to the second month between the treatment and placebo groups, accounting for the placebo effect. Notably, this index was significant for the visuoconstructional and NUCOG variables.
The results between the two groups did not differ from one month to two months, indicating the follow-up period. There was no interaction between time and treatment groups, suggesting the absence of a modifier effect or interaction effect.
Table 3 presents the parameters of the SAPS, the SANS, and the NUCOG variables at three time points: The beginning of the intervention, the end of the first month, and the end of the second month.
| Variables and Months | Β [95%CI] | P-Value |
|---|---|---|
| SAPS | ||
| 1 | 0.47 [0.14; 0.81] | 0.006 a |
| 2 | 1.1 [0.19; 1.99] | 0.018 a |
| SANS | ||
| 1 | 0.07 [-0.03; 0.18] | 0.163 |
| 2 | 0.58 [-0.09; 1.25] | 0.087 |
| Attention | ||
| 1 | 0 | - |
| 2 | -0.41 [0.21; 0.11] | 0.545 |
| Memory | ||
| 1 | 0.043 [-0.03; 0.11] | 0.278 |
| 2 | -0.04 [-0.26; 0.17] | 0.687 |
| Executive function | ||
| 1 | -0.1 [-0.26; 0.06] | 0.226 |
| 2 | -0.16 [-0.47; 0.15] | 0.306 |
| Visuospatial | ||
| 1 | -0.27 [-0.51; -0.02] | 0.031 a |
| 2 | 0.69 [-1.1; -0.24] | 0.003 a |
| Language | ||
| 1 | 0 | - |
| 2 | 0 [-0.17; 0.17] | 0.997 |
| Total | ||
| 1 | -0.27 [-0.59; 0.04] | 0.090 |
| 2 | -0.84 [-1.45; -0.22] | 0.009 a |
Scale for Assessment of Positive Symptoms, Scale for Assessment of Negative Symptoms, And Neuropsychiatry Unit Cognitive Assessment Tool Variables and Their Fields
In summary, treatment with NAC had a positive and significant impact on the SAPS, NUCOG indices, and GSH test results, as shown in Figures 2 - 4. Follow-up of drug use in the second month demonstrated a positive effect on the NUCOG Index, particularly the visuoconstructional variable. However, continued consumption did not significantly affect the SAPS Index. Considering the limited side effects observed during the study periods, NAC is evaluated as a positive and practical treatment option. The relationship between the generation of antipsychotic drugs consumed and the total cognitive score at the one-month and two-month stages was assessed by controlling for confounding variables such as age, sex, and treatment group. No significant differences were found in the NUCOG, SAPS, and SANS scores between users of first- and second-generation antipsychotic drugs.
5. Discussion
Schizophrenia is a debilitating condition characterized by positive and negative symptoms, as well as cognitive impairment. While antipsychotic drugs have shown some effectiveness in treating positive symptoms, there are currently no robust treatments for negative or cognitive symptoms (19). Recent research suggests that antioxidant therapy supplementation may help alleviate these symptoms. Redox dysregulation is believed to play a crucial role in the pathophysiology of schizophrenia, with reduced levels of the antioxidant GSH in the brain linked to oxidative stress and schizophrenia (20). N-acetylcysteine, a precursor of GSH, is a nutraceutical and novel therapeutic approach that has shown potential in alleviating symptoms. Multiple evaluations of NAC as a psychiatric treatment have demonstrated its potential as an adjuvant therapy for several mental disorders (21-25).
Neuronal deterioration in specific brain regions is considered a principal factor in the development of major depressive disorders. Therefore, protecting and maintaining the standard structure and function of neurons might be a potential therapeutic strategy for treating mood disorders such as depression (26, 27). In a study, Fan et al. reported that NAC inhibited neuronal injury by reducing oxidative stress and exerting antidepressant effects. Specifically, antioxidant enzyme activity was significantly decreased in the hippocampal CA1 region of depressive rats. N-acetylcysteine treatment (300 mg/kg, i.p.) produced neuroprotective effects against mitochondrial oxidative stress injuries and oxidative DNA damage in CA1 neurons. Moreover, NAC alleviated neuronal injury from neuroinflammation and apoptosis in depressed rats, associated with reductions in dendritic spine atrophy and synapse deficits. These results suggest the potential involvement of oxidative stress in depression generation, with NAC's antidepressant-like effects possibly involving reductions in oxidative stress leading to neuronal deterioration. Such neuroprotective effects of NAC may indicate a potential therapeutic strategy for stress-related depression (28).
Zheng et al. conducted a meta-analysis on NAC for schizophrenia, finding preliminary evidence of its potential for negative symptoms (29). In our study, NAC significantly improved cognitive function and positive symptoms of schizophrenia, but no substantial improvement in negative symptoms was detected. Similarly, a randomized controlled trial by Neill et al. found that NAC (2 grams per day) did not significantly improve negative symptoms, overall cognition, or QOL over one year of treatment (30). Another study involving 42 patients with chronic schizophrenia and a PANSS negative subscale score of 20 or greater showed that NAC-treated patients improved significantly more in PANSS total and negative subscale scores than the placebo group, though this difference was insignificant for positive and general psychopathology subscales (14). Sepehrmanesh et al. reported significant improvements in positive and negative PANSS subscales in NAC-treated patients, with declines in general and total PANSS scores over time, while placebo group scores increased. Improvements were observed in cognitive functions such as attention, short-term and working memory, executive functioning, and processing speed (31).
The controversies in the literature may be due to varying treatment protocols, NAC dosages, assessment tools, and follow-up periods. The most significant limitations of our study were the relatively small sample size and patient compliance with medication usage.
5.1. Conclusions
Our study demonstrated that the addition of 600 milligrams of NAC to the standard treatment regimen of chlorpromazine for schizophrenia significantly improved positive symptoms and cognitive functions, although it did not enhance negative symptoms. Considering the limited side effects observed during the study periods, NAC can be regarded as an effective adjunctive treatment for positive symptoms and cognitive functions in schizophrenia.