1. Background
Fibromyalgia is one of the most common chronic disorders that affects 3 - 6% of the world’s population, particularly women (75 - 90%) (1). This disease is characterized by chronic widespread musculoskeletal pain accompanied by fatigue, sleep disorders, cognitive impairment, and multiple somatic symptoms. However, it adversely affects several other aspects of the patient’s life, including sexual function and sociomedical acceptance (2). Invalidation and fibromyalgia-associated comorbidities significantly impair the patient's quality of life and may result in unemployment and withdrawal from social life (3). Delayed diagnosis because of the absence of specific diagnostic laboratory tests or biomarkers results in a remarkable economic burden on the healthcare system, patients, and their families (4). Direct and indirect costs of fibromyalgia further increase the true burden of fibromyalgia (5). Pregabalin, duloxetine, and milnacipran are the only FDA-approved pharmacologic treatments for fibromyalgia (6). However, they are associated with several side effects, which many patients cannot tolerate (6). On the other hand, drug discontinuation is associated with a high risk of relapse (7). Therefore, developing non-pharmacologic treatments for fibromyalgia is of critical importance.
Oxidative stress is regarded as the imbalance between the production and degradation of reactive oxygen/nitrogen species (8). The role of oxidative stress is established in the physiopathology of various chronic diseases, including fibromyalgia (9, 10). In a study by Eisinger et al., protein peroxidation was prominent in fibromyalgia patients, while no significant difference was found in antioxidant status between fibromyalgia patients and control subjects (11). In another study by Bagis et al., fibromyalgia patients showed significantly elevated levels of malondialdehyde (a marker of oxidative damage) and reduced activity of superoxide dismutase (a marker of antioxidant capacity) (12). In the study of Altindag and Celik, plasma total antioxidant capacity (TAC) was significantly lower in fibromyalgia patients compared to healthy controls. In contrast, the total plasma peroxide level and oxidative stress index (OSI) were significantly higher in fibromyalgia patients than in controls (13).
A mutual interplay has also been suggested between oxidative stress and fibromyalgia symptoms. In this respect, fibromyalgia symptoms, such as pain, depression, and sleep disturbance, enhance oxidative stress (14, 15). This increased oxidative stress negatively affects various cellular structures, such as DNA, lipids, proteins, and lipoproteins, further expanding the fibromyalgia burden in a vicious cycle (16). A direct association has been demonstrated between oxidative stress and the severity of fibromyalgia symptoms (17-19).
Cognitive-behavioral therapy (CBT) is a psychotherapeutic approach that helps people identify their destructive and disturbing thinking patterns, thereby contributing to considerable improvements in pain-triggered behaviors, coping strategies, self-efficacy, and physical functioning (20). Cognitive-behavioral therapy is acknowledged as a valuable non-pharmacological treatment for fibromyalgia, and its efficacy in controlling fibromyalgia symptoms, such as pain and sleep disturbance, has been approved in several studies (21, 22). Accordingly, we hypothesized that the improvement of fibromyalgia symptoms with CBT could result from the amelioration of oxidative balance. No prior research has investigated the potential impact of CBT on oxidative balance in fibromyalgia patients.
2. Objectives
In this randomized controlled trial, we aimed to investigate whether CBT intervention could influence the serum markers of oxidative balance, including total oxidant status (TOS) and TAC, in fibromyalgia patients.
3. Methods
3.1. Study Design
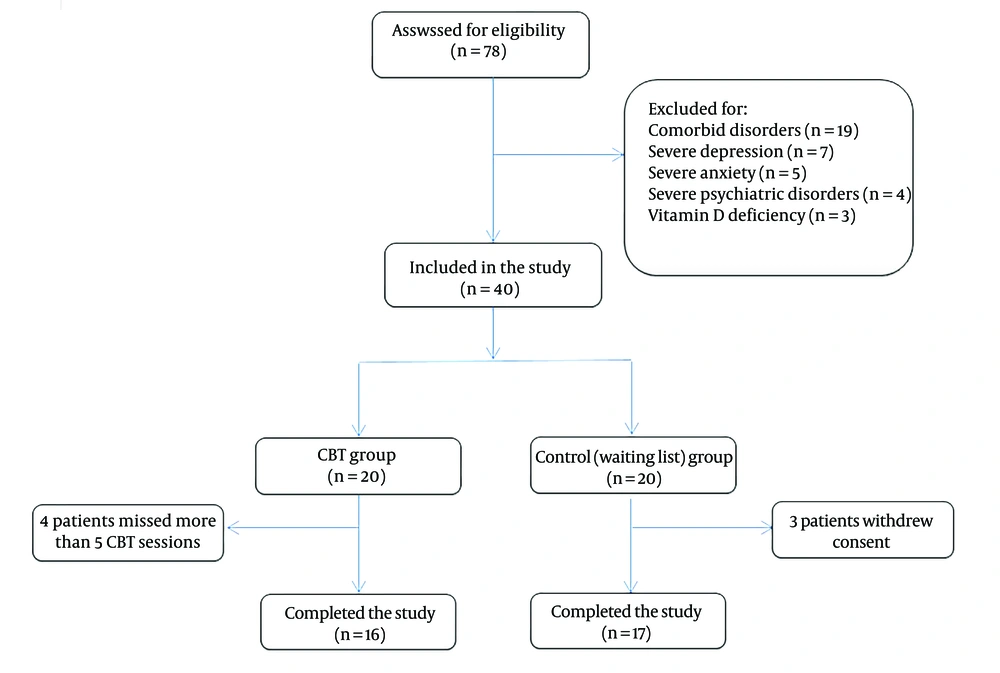
This study was approved by our institute’s review board and Ethics Committee under the code IR.IUMS.REC.1398.709. Patients provided written informed consent before participation in the study. The protocol of the study was registered in the Iranian Registry of Clinical Trials under the code IRCT2016120530871N3. The participants were selected among the fibromyalgia patients referred to the rheumatology clinic of the Shafa Orthopedic Hospital. Inclusion criteria were female gender, age of 18 - 65 years, and a definitive diagnosis of fibromyalgia according to the 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria announced by the American College of Rheumatology (ACR) (23). Exclusion criteria included the presence of comorbid conditions affecting the serum oxidant status and antioxidant capacity, including any type of arthritis, metabolic disorders, and infectious conditions. Patients with severe psychiatric disorders, depression, or anxiety, making them incapable of following behavioral instructions, were also excluded. A clinical psychologist diagnosed these severe psychiatric disorders through detailed history taking and clinical evaluations according to the DSM-5 (diagnostic and statistical manual of mental disorders, fifth edition) diagnostic classification (24). Severe depression or anxiety was detected according to the Beck scale score for depression and anxiety. A score of 30-63 was regarded as severe depression or anxiety. The Persian translations of the Beck Depression Inventory-II (BDI-II) (25) and Beck Anxiety Inventory-II (BAI-II) were used in this regard (26). Patients with vitamin D deficiency were excluded because of its confounding effects on the association between oxidative balance and fibromyalgia (27). Figure 1 demonstrates the flow diagram of patient enrollment according to the inclusion and exclusion criteria.
3.2. Randomization and Blinding
Fibromyalgia patients were randomly assigned to either the case (CBT) group or the control (waiting list) group using the parallel assignment and a computer-generated randomization list (rand function of Excel software). In this respect, a random number list was created between 1 and 40. The first 20 random numbers were assigned to the CBT group, and the following 20 random numbers were assigned to the waiting list group (1:1 ratio). Then, the numbers were sorted from the smallest to the largest, and consecutive patients were assigned to either the CBT or waiting list group based on the order of referral. Individuals responsible for laboratory evaluations and statistical analyses were blinded to the patient’s allocation to reduce the risk of bias. However, complete blinding was impractical as we used the patients on the CBT waiting list as the control group, so these patients and researchers knew the waiting list would receive no treatment before the start of the CBT course.
3.3. Intervention
The CBT group received traditional face-to-face CBT, as previously described (28), with some minor modifications. The CBT program was offered twice weekly over a period of ten weeks (a total of 20 sessions) with the following topics: extraction of disturbing thoughts (2 sessions), explaining the interaction between troublesome thoughts and behaviors (2 sessions), introducing inaccurate thinking patterns (four sessions), and instructing appropriate thinking patterns combined with behavioral CBT components, including relaxation training, activity pacing, distraction activities, and healthy lifestyle/habits (12 sessions). The CBT course was administered by a clinical psychotherapist. We excluded patients who missed more than five CBT sessions. The patients in the control group were selected from the patients who were on the waiting list for CBT. They were asked not to take any pharmacologic treatment while they were waiting for the beginning of the CBT course.
3.4. Data Collection and Analysis
A volume of 5 mL blood samples was taken from the patients before the start of the study and immediately after the end of the CBT course in the intervention group. In the control group, blood samples were taken before enrolment and ten weeks after that. All the samples were collected in the early morning and after fasting for 12 hours. Sera were extracted and kept in a -80ºc refrigerator for later analyses. The primary outcome measures were the serum levels of TOS and TAC. For TAC assessment, under an acidic condition, the oxidizing material in the sample oxidized the ferrous ion (Fe2+) to the ferric ion (Fe3+), which then reacted with xylenol orange to produce a blue-purple complex. Maximum absorption was measured at 590 nm, which was proportional to the content of oxidative substances in the serum sample (Zellbio Gmbh, Germany, ZB-TAC-96A). The TAC concentration was presented as mmol TROLOX Eq/L. The detection limit of the TAC assay was 5.91 µM, and its sensitivity was 8.06 µM. Also, TOS concentration was presented as mmol H2O2 Eq/L. The detection limit of the TOS assay was 3.86 µM, and its sensitivity was 5 µM (Zellbio Gmbh, Germany, ZB-TOS-96A).
3.5. Sample Size
The sample size was calculated using G*power 3.1.9.2 software, and the mean and standard deviation of serum TAC in fibromyalgia patients and healthy controls were extracted from the study of Altindag and Celik (13). Regarding an effect size of 1.3, a power of 95%, and a significance level of 5%, 16 patients in each group were considered sufficient to detect a clinically significant mean difference between the two study groups using a two-sided independent t-test. Considering a drop-out rate of 20%, a total of 20 patients were included in each study group.
3.6. Statistical Analysis
SPSS for Windows version 16 (SPSS Inc., Chicago, Ill., USA) was used for statistical analyses. Data were presented as mean ± standard deviation or frequency and percentage. The normality of distribution was examined using the Kolmogorov–Smirnov test. The Wilcoxon signed-rank test was used for before/after comparisons, and the Mann-Whitney U Test was used to compare mean values between the two study groups. Spearman's correlation was used to disclose potential correlations between variables. A P-value of <0.05 was considered statistically significant.
4. Results
4.1. Demographic Characteristics of Patients
Sixteen patients in the CBT group and 17 patients in the control group completed the study. The mean age of the patients was 45.5 ± 7.5 years in the CBT group and 47.5 ± 6.4 years in the control group (P = 0.43). The mean disease duration was 23.2 ± 11.5 months in the CBT group and 21.6 ± 12 months in the control group (P = 0.29). The mean BMI was 26.8 ± 6.2 kg/m2 in the CBT group and 27.1 ± 7 kg/m2 in the control group (P = 0.51). No significant difference was also observed in other baseline characteristics between the two study groups, including the menopausal status, educational level, and serum vitamin D levels (Table 1).
| Variables | CBT Group (n = 16) | Control (Waiting List) Group (n = 17) | P-Value |
|---|---|---|---|
| Age (y) | 45.5 ± 7.5 | 47.5 ± 6.4 | 0.43 |
| BMI (kg/m2) | 26.8 ±6.2 | 27.1 ± 7 | 0.51 |
| Job | 0.66 | ||
| Unemployed | 11 (68.7) | 13 (76.5) | |
| Employed | 5 (31.3) | 4 (23.5) | |
| Menopause | 0.72 | ||
| Pre | 12 (75) | 14 (82.4) | |
| Post | 4 (25) | 3 (17.6) | |
| Academic education | 0.38 | ||
| Yes | 9 (56.3) | 7 (41.2) | |
| No | 7 (43.7) | 10 (58.8) | |
| Serum vitamin D level (ng/mL) | 36.5 ± 11.7 | 35.8 ± 12.3 | 0.48 |
| Duration of disease (mo) | 23.2 ± 11.5 | 21.6 ± 12 | 0.29 |
| Beck Depression Inventory score | 18.2 ± 8.6 | 18.6 ± 8.9 | 0.71 |
| Beck Anxiety Inventory score | 21.5 ± 7.6 | 21± 7.2 | 0.66 |
a Values are presented as mean ± SD or No. (%).
4.2. Within-Group Analysis
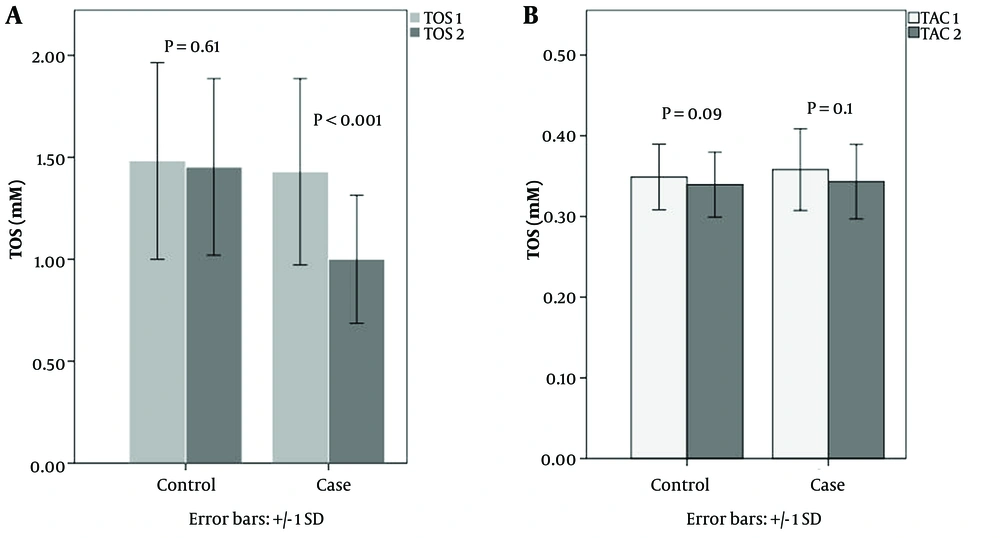
In the CBT group, the mean TOS was 1.43 ± 0.46 mM before the CBT and 1 ± 0.31 mM after the end of the CBT course. This difference was statistically significant (P < 0.001). Also, the mean TAC was 0.36 ± 0.05 mM before the start of the CBT course and 0.34 ± 0.04 mM after the end of the CBT course. This difference was not statistically significant (P = 0.1).
In the control (waiting list) group, the mean TOS was 1.48 ± 0.48 mM at the baseline and 1.45 ± 0.43 mM at the end of the study. Mean TAC was 0.35 ± 0.04 mM at the start of the study and 0.34 ± 0.05 mM at the end of the study. Neither of the differences was statistically significant (P = 0.61 and P = 0.09, respectively).
4.3. Between-Group Analysis
The mean change in TOS was 0.43 ± 0.3 mM in the CBT group and 0.03 ± 0.2 mM in the control group. This difference was statistically significant (P < 0.001). The mean change in TAC was 0.015 ± 0.03 mM in the CBT group and 0.01 ± 0.02 mM in the control group, showing no statistically significant difference (P = 0.57, Figure 2).
4.4. Correlation Analysis
The Beck Depression Inventory score was positively correlated with TOS (r = 0.341, P = 0.02) but not with TAC (r = 0.011, P = 0.89), and the Beck Anxiety Inventory score was significantly correlated with neither TOS (r = 0.077, P = 0.43) nor TAC (r = -0.052, P = 0.55). The serum vitamin D level was not significantly correlated with TOS (r = 0.063, P = 0.52) or TAC (r = 0.102, P = 0.17). The age of fibromyalgia patients was not significantly correlated with TOS (r = 0.091, P = 0.31) or TAC (r = 0.085, P = 0.41). Also, disease duration was significantly correlated with neither TOS (r = 0.076, P = 0.45) nor TAC (r = -0.051, P = 0.62). In addition, no significant correlation was observed between other demographic characteristics of the patients and oxidative balance indices, including BMI, employment status, menopausal status, and educational status.
5. Discussion
In this study, we evaluated if CBT could affect serum TAC and TOS in fibromyalgia patients. Based on our results, The mean of serum TAC did not significantly change after the CBT program. However, the mean serum TOS showed a significant reduction at the end of the CBT course. Limited evidence is available regarding the impact of therapeutic interventions on oxidative balance in fibromyalgia patients and those with similar disorders such as depression. Cetinkaya et al. aimed to find whether or not there was a difference between the oxidative balance of fibromyalgia patients and healthy controls and how balneotherapy could affect this balance, as well as the clinical symptoms, such as pain, depression, and quality of life of these patients. Thirty-five female fibromyalgia patients and 35 healthy females were included in the recent study; the fibromyalgia patients received 15 sessions of balneotherapy, and the laboratory and clinical parameters of the patients were reevaluated afterward. According to their results, serum TAS (total antioxidant status) was not significantly different between fibromyalgia patients and controls; however, serum TOS and OSI levels were significantly higher in fibromyalgia patients. Balneotherapy led to a significant decrease in serum TOS and OSI levels in fibromyalgia patients, but it showed no significant effect on serum TAS. Besides, all clinical parameters significantly improved with balneotherapy (29). In the present study, we evaluated the effect of CBT, and not balneotherapy, on the oxidative balance of fibromyalgia patients. Although CBT and balneotherapy are two different therapeutic strategies, they both aim at alleviating stress-related symptoms and improving cognitive function (20, 30). Therefore, the same change in oxidative balance could be expected for CBT and balneotherapy. Similar to the study of Cetinkaya et al., we observed that serum TOS was significantly reduced following the CBT intervention (29). Also, we observed that CBT had no significant impact on serum TAC. Theoretically, the capacity of cells to produce antioxidants is limited, and at some points, no further antioxidants can be generated. If this hypothesis is correct, the antioxidant level could be correlated with the severity of the disease. At the beginning of the disease, cells can still increase their antioxidant production, and CBT intervention could enhance TAC. However, patients with a long time from their diagnosis might have already used their full capacity for antioxidant production, where therapeutic programs such as CBT cannot further improve TAC. The clinical implications of these results suggest that the focus should be on reducing TOS or using synthetic antioxidants to increase TAC in fibromyalgia patients, thereby ameliorating the oxidative balance. Consistent with the study of Cetinkaya et al., clinical indices of fibromyalgia, including the fibromyalgia impact questionnaire (FIQ) score and widespread pain index (WPI), were significantly reduced in the CBT group. However, as these data were presented in our previous report, they were not included in this study to avoid duplication (31).
Cumurcu et al. evaluated the impact of 12 weeks of antidepressant treatment on TAC and TOS in 57 patients with major depressive disorder and 40 healthy controls. Before the intervention, TOS and OSI were significantly higher, while TAC was significantly lower in the depression group. Three months of antidepressant therapy significantly decreased TOS and OSI and significantly increased TAC. The disease severity was directly correlated with TOS and OSI and inversely correlated with TAC (32). By contrast, we did not observe any significant effect of CBT on TAC levels in fibromyalgia patients. This difference could be attributed to the different patient populations in the two studies (depression vs. fibromyalgia). In addition, Cumurcu et al. evaluated the effect of a pharmacologic treatment on oxidative balance, while we used a non-pharmacologic treatment in our study.
The present study showed improved oxidative balance in fibromyalgia patients following CBT treatment. This improvement could be attributed to the improvement of fibromyalgia symptoms following CBT-associated cognitive changes. Symptoms such as insomnia and depression can disrupt the oxidant-antioxidant balance (14, 15), and poor sleep quality has been shown to impair the mitochondrial electron transport chain, thereby increasing the production of reactive oxygen species, leading to oxidative stress (33). A better sleep quality potentially improves oxidative balance. Interestingly, oxidative balance has been reported to improve following a boost in sleep quality and the subside of depression in patients with psychiatric disorders undergoing CBT (15)
The present study was not without limitations. As the main limitation, the TAC assay is not accurately indicative of “total" antioxidant capacity because it excludes the endogenous enzymatic activity of enzymes such as catalase and glutathione (34). Therefore, complementary evaluations on endogenous enzymatic activities in future research can help better understand the therapeutic effects of CBT on oxidative balance. The small number of patients could be regarded as the other limitation of this study, which did not allow us to conduct multivariate data analyses. Therefore, future studies on larger populations are required to better explain the impact of CBT on the oxidative balance in fibromyalgia patients. Finally, we used patients on the CBT waiting list as the control group. This design could have increased the risk of bias mostly due to a lack of blinding.
5.1. Conclusions
Serum TOS significantly reduced following the CBT intervention in fibromyalgia patients, suggesting that one of the therapeutic mechanisms of CBT is mediated through balancing oxidative stress, further supporting its applicability for treating fibromyalgia. Accordingly, CBT could be regarded as a single or joint treatment for fibromyalgia patients. Complementary well-designed studies on a higher number of participants could better demonstrate the association between CBT and oxidative balance in fibromyalgia patients.