1. Background
Cannabis is the most frequently used illegal substance in the world (1). Scientifically referred to as Cannabis sativa and belonging to the Cannabaceae family, it is commonly known as marijuana or hemp. Throughout many regions of the world, cannabis has been utilized as a medicinal herb for numerous centuries, with a rich historical background (2). Scientific studies have indicated that cannabis exhibits positive effects by inhibiting tumor growth and alleviating nausea. Additionally, it influences behavioral traits such as impulsivity, compatibility, and sensitivity to heat. Cannabis can also modulate mood, appetite, and sexual activity. However, it is worth noting that the presence of cannabinoid compounds, particularly delta-9-tetrahydrocannabinol (THC), contributes to its rewarding properties, resulting in pleasurable experiences, and can potentially lead to addiction (3).
The primary active compound found in cannabis, THC, interacts with specific receptors in the brain known as cannabinoid receptors. This interaction results in temporary feelings of euphoria, relaxation, and relief. However, it is important to note that THC can also have detrimental effects on cognitive functions, including memory, learning, concentration, and body coordination (4, 5). According to the World Drug Report of 2018, over 269 million people worldwide have used various forms of cannabis, including marijuana, hashish, cannabis resin, and cannabis oil. Cannabis is recognized as the most widely used illicit substance in the world (6). In 2017, the number of cannabis users was reported to be 238 million (7), and in 2015, it was 183 million (1). The main symptoms of cannabis withdrawal are anxiety, irritability, depressed mood, restlessness, disturbed sleep, gastrointestinal symptoms, and decreased appetite. Most symptoms begin during the first week (8).
Transcranial direct current stimulation is a non-invasive method of neuromodulation that involves the application of a weak direct electric current to cortical areas. This technique aims to either facilitate or inhibit neural excitability in the targeted regions (9).
Over the past decade, tDCS has emerged as a well-researched and safe non-invasive technique for modulating cortical excitability. This method offers a non-invasive, cost-effective alternative without significant risks. It involves the administration of a direct electrical current to the brain, leading to modifications in the resting membrane potential of cortical neurons and subsequent alterations in cortical excitability (10). In this method, a weak electrical current of 0.2 - 0.5 milliamperes is used to stimulate targeted brain areas to achieve therapeutic effects (11).
By connecting two electrodes, an anode and a cathode, to specific locations on the surface of the skull, a weak and direct electrical current is applied, thereby stimulating the underlying neurons. Stimulation through the anode increases brain excitability, whereas stimulation through the cathode decreases brain excitability (12). The significant role of the DLPFC in cognitive functions, specifically executive functions like working memory, goal-directed behavior, attention, and inhibitory control, has garnered substantial interest among researchers. Prioritizing this brain region holds promise for potentially enhancing cognitive performance and disrupting maladaptive behaviors (10, 13). Positive effects of electrical stimulation on the DLPFC have been reported in terms of working memory, information processing, attention and focus, emotional processing, and problem-solving (10, 13).
Moreover, research findings suggest the efficacy of direct electrical stimulation administered to the skull in diminishing symptoms associated with depression and social anxiety (14, 15). When elucidating the impact of tDCS on the left DLPFC, it can be described that the application of tDCS induces alterations in neuronal excitability and modulates the membrane potential of superficial neurons, either depolarizing or hyperpolarizing them (14, 15). This modulation subsequently leads to an increase or decrease in the firing of brain cells (14).
Neuroimaging studies have revealed that the DLPFC plays a neural role not only in craving and substance abuse but also in mood and cognitive disorders (16). Clinical observations have additionally indicated the involvement of the DLPFC in attentional control and its role in regulating processes related to substance craving and decision-making (17).
Hence, the DLPFC can serve as a viable target for stimulation aimed at decreasing the intensity of cravings and minimizing the risk of relapse among individuals struggling with substance abuse (18). Many individuals who discontinue substance use often encounter cravings or relapses, with the highest probability of relapse occurring within the initial 90 days following the initiation of abstinence (18). Depression and working memory can be influenced by cannabis use, and transcranial direct current stimulation (tDCS) may have potential effects on both (19).
2. Objectives
This study sought to examine the effectiveness of tDCS on the DLPFC in reducing cravings, improving depression, and enhancing working memory among individuals with cannabis use disorder. The motivation behind this investigation stemmed from recognizing the inadequacy of conventional treatment approaches for substance use disorders, as well as the considerable significance and inherent difficulties associated with managing cravings or temptations faced by therapists in this particular field.
3. Methods
3.1. The Participants and Research Design
The present study employed a randomized, double-blind, sham-controlled design. The target population consisted of men with cannabis addiction who sought treatment at substance abuse clinics in Karaj city. The sample size for the study was 50 participants, randomly assigned to two groups of 25 individuals each.
Individuals who met the study inclusion criteria were selected using convenience sampling. The inclusion criteria consisted of the following: Age range between 20 and 40 years, absence of psychiatric disorders such as bipolar disorder, schizophrenia, or major depression, absence of physical illnesses such as malignancies, diabetes, recent cardiac or cerebral infarction, and active seizures, absence of other substance dependencies and abuses except for smoking, and presence of cannabis dependence based on DSM-5 criteria. The exclusion criteria included sensitivity to the tDCS method at any point during the study and experiencing adverse effects from the aforementioned method, positive urine tests for two consecutive times, lack of interest in continuing the study, the onset of diseases such as malignancies or diabetes during the study, and irregular attendance at therapy sessions and interventions.
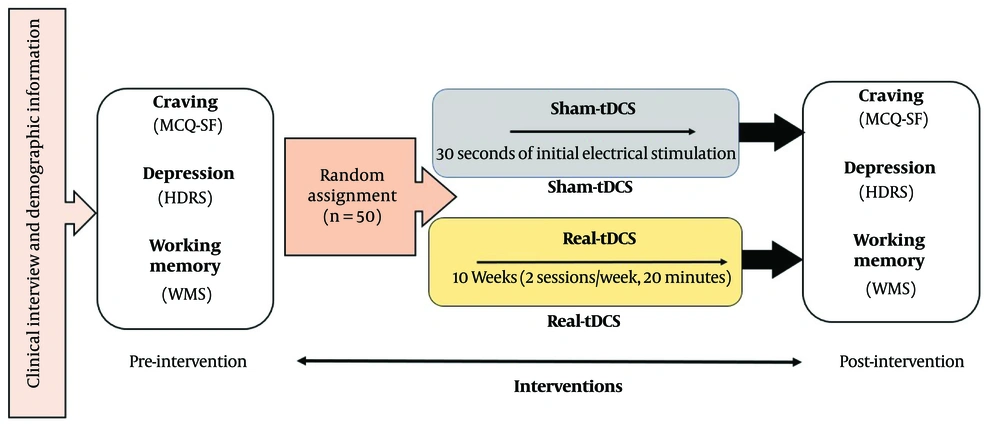
After selecting the samples and obtaining informed consent, the participants underwent interviews and clinical evaluations, and a urine test for marijuana was conducted (Rozhan Teb company). Immediately, pre-tests were administered, including the Hamilton Depression Rating Scale (HDRS), the Wechsler Memory Scale (WMS), and the Marijuana Craving Questionnaire-Short Form (MCQ-SF) to measure depression, working memory, and cannabis craving, respectively. The patients were then coded by the operator of the tDCS device, and without the knowledge of the treatment team and patients, they were assigned to two equal groups: Real tDCS and sham tDCS. The sham tDCS was administered by providing electrical stimulation for only 30 seconds at the beginning of the session, after which no stimulation intensity was applied to the patient.
The operator remained unaware of the treatment outcomes, as well as the patients' test results and urine screenings. After the completion of the tDCS sessions, all patients immediately underwent post-assessments, including the MCQ-SF cannabis craving questionnaire, the Hamilton Depression Scale, and the Wechsler Memory Scale (WMS), as part of the post-test evaluation (Figure 1). The trial is registered and approved in the Iranian Registry of Clinical Trials as of 2022-09-12, with the registration reference IRCT20220823055784N1.
Experimental procedure. The experiments were conducted in a randomized, double-blind, parallel-group design. MCQ-SF = marijuana urine tests, Marijuana Craving Questionnaire-Short Form; tDCS = Transcranial Direct Current Stimulation; HDRS = Hamilton Depression Rating Scale; WMS = Wechsler Memory Scale.
After data collection, the operator provided the tDCS Real and Sham codes to the researcher for data analysis purposes. All patients who underwent the intervention received standard psychological treatments in accordance with ethical considerations. No patient was deprived of standard treatment, and the research protocol was approved by the Iran University Ethics Committee (IR.IUMS.REC.1400.1147).
3.2. Transcranial Direct Current Stimulation
The NEUROSTIM-2 device by Medina TebGostar Company was used for tDCS. The number of tDCS sessions was determined to be 20 based on similar studies (18, 20). The sessions were conducted twice a week, with each session lasting 20 minutes. The stimulation intensity was set at 2 milliamperes, with a fall and rise frequency of 30 seconds. The electrode placement followed the standard 10 - 20 EEG system, specifically targeting the F3 and F4 positions, which are relevant to DLPFC stimulation. This protocol has been found effective in reducing craving and improving depression, according to relevant studies (21, 22).
Patients receiving tDCS also underwent standard psychological treatments in accordance with ethical considerations, ensuring no patient was deprived of standard care. The NEUROSTIM-2 device by Medina TebGostar was employed for the transcranial electrical stimulation. The sessions were conducted twice a week, each lasting 20 minutes, with a current intensity of 2 milliamperes and a fall and rise frequency set at 30 seconds. The single-channel configuration at the F3 and F4 standard points, targeting the DLPFC region, has been shown to be effective in reducing cravings and improving depression.
3.3. Tools
3.3.1. Marijuana Craving Questionnaire-Short Form (MCQ-SF)
The MCQ (Marijuana Craving Questionnaire) is a 47-item questionnaire designed to assess the desire for marijuana across four dimensions: Compulsion, Emotion, expectancy, and purposefulness. Heishman et al. developed a 12-item version of the MCQ for use in research and clinical settings, selecting three items from each of the four factors that demonstrated the highest internal consistency. The Cronbach's alpha coefficient and the mean inter-item correlation (in parentheses) for the respective dimensions were determined to be 0.61 (0.34), 0.75 (0.51), 0.72 (0.46), and 0.84 (0.64) (23).
In another study conducted by Chauchard et al., the Cronbach's alpha coefficient and the mean inter-item correlation were found to be 0.87 and 0.44, respectively (24). The results from both studies indicate the reliability and validity of the questionnaire. This 12-item questionnaire is based on a 7-point Likert scale (ranging from 1, strongly disagree, to 7, strongly agree). Participants select their responses based on the importance they attribute to each question.
3.3.2. Hamilton Depression Rating Scale (HDRS)
The Hamilton Depression Rating Scale (HDRS) is a widely used tool for evaluating the severity of depressive symptoms through a clinical interview. Developed by Hamilton (25), it consists of 27 items that are rated by the interviewer using a Likert scale. The HDRS has demonstrated concurrent and construct validity, as its scores have shown agreement with scores from structured clinical interviews. It has a high sensitivity in detecting positive cases of depression, with a sensitivity rate of 87% or higher. In multiple studies, the HDRS has shown the highest sensitivity and specificity at a clinical cutoff point of 17, with a sensitivity of 62.4% and a specificity of 92% (26).
3.3.3. Wechsler Memory Scale (WMS)
The Wechsler Memory Scale (WMS) is a widely used psychological assessment tool designed to measure different aspects of memory functioning in individuals. Developed by David Wechsler, the WMS assesses various memory domains, such as immediate and delayed recall, recognition, and working memory. The internal consistency reliability of the WMS is very high, with a coefficient alpha of 0.90. The test-retest reliability of this scale is also high, with coefficients ranging from 0.80 to 0.89 (27). In a study conducted in Iran, the reliability of the WMS was found to be 0.74 using the Cronbach's alpha method (28).
3.4. Data Analysis
For data analysis, SPSS 22 software was used. A one-way analysis of variance (ANOVA) was employed to compare age and education, while an independent t-test was used to compare marital status between the two study groups. Paired t-tests were conducted to compare pre- and post-test scores on the MCQ-SF craving measures, the Hamilton Depression Rating Scale (HDRS), and the Wechsler Memory Scale. Additionally, independent t-tests were used to compare the mean percentage changes in craving for cannabis, depression, and working memory between the study groups.
4. Results
Table 1, based on t-test and χ2 test, displays the demographic characteristics of the participants before the intervention. As observed in Table 1, there were no statistically significant differences in terms of age, education, and marital status between the two groups.
| Variables | tDCS (n = 25) Real | tDCS (n = 25) Sham | P-Value |
|---|---|---|---|
| Age | 29.44 ± 5.91 | 29.60 ± 6.36 | 0.43 |
| Education (y) | 12.16 ± 2.99 | 11.36 ± 3.99 | 0.93 |
| Marital status | 0.51 | ||
| Single | 15 (51.7) | 14 (48.3) | |
| Married | 10 (47.6) | 11 (52.4) |
Abbreviation: tDCS, Transcranial Direct Current Stimulation.
a Values are expressed as mean ± SD or No. (%).
4.1. Impact of Transcranial Direct Current Stimulation on Cannabis Craving
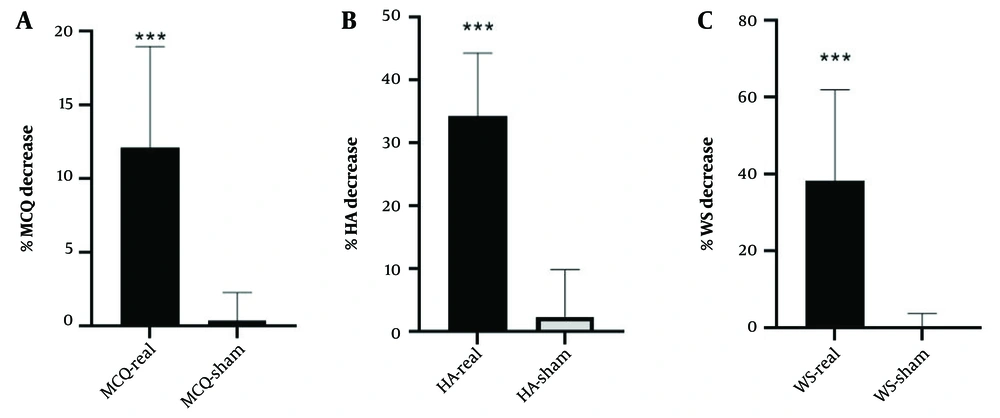
Based on Table 2 and the results of the MCQ-SF test, participants who received real tDCS showed a significant reduction in their craving for cannabis. Additionally, according to the results in Table 3 and Figure 2, participants who received real tDCS experienced a 12.10% reduction in their cannabis craving scores on the MCQ-SF, while in the sham tDCS group, this reduction was only 0.37%. This difference was statistically significant.
| Variables | tDCS (n=25) Real | t | P-Value | tDCS (n=25) Sham | t | P-Value | ||
|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | |||||
| MCQ-SF | 47.92 ± 12.68 | 42.36 ± 12.29 | 7.17 | < 0.0001 | 53.12 ± 3.19 | 52.92 ± 3.27 | 1.00 | 0.327 |
| HDRS | 23.04 ± 4.44 | 15.40 ± 4.49 | 27.42 | < 0.0001 | 20.32 ± 4.13 | 19.76 ± 3.80 | 1.66 | 0.110 |
| WMS | 49.32 ± 11.22 | 66.68 ± 11.77 | -19.72 | < 0.0001 | 53.36 ± 14.90 | 53.72 ± 15.20 | -1.12 | 0.273 |
Abbreviations: M, mean; SD, standard deviation; WMS, Wechsler Memory Scale; HDRS, Hamilton Depression Rating Scale; MCQ-SF, Marijuana Craving Questionnaire-Short Form.
a Values are expressed as mean ± SD.
4.2. The Impact of Transcranial Direct Current Stimulation on Depression
According to Table 2 and the results of the Hamilton Depression Rating Scale (HDRS), participants who received real tDCS showed a significant reduction in their depression levels. Additionally, based on Table 3 and Figure 2, participants in the real tDCS group demonstrated a 34.19% improvement in their HDRS scores, while those in the sham tDCS group showed an improvement of only 2.29%. This difference was statistically significant.
| Variables | tDCS (n = 25) Real | tDCS (n = 25) Sham | t | df | P-Value |
|---|---|---|---|---|---|
| MCQ-SF | -12.10 ± 6.85 | -0.37 ± 1.89 | 8.26 | 48 | < 0.0001 |
| HDRS | -34.19 ± 10.10 | -2.29 ± 7.55 | 11.96 | 48 | < 0.0001 |
| WMS | 38.33 ± 23.58 | 0.51 ± 3.18 | -7.95 | 48 | < 0.0001 |
a Values are expressed as mean ± SD.
Abbreviations: M, mean; SD, standard deviation; WMS, Wechsler Memory Scale; tDCS, transcranial direct current stimulation; HDRS, Hamilton Depression Rating Scale; MCQ-SF, Marijuana Craving Questionnaire-Short Form. percentage of changes: (Post-test-Pre-test)/ Pre-test ×100.
4.3. The Impact of tDCS on Working Memory
According to Table 2 and the results of the working memory test by WMS, participants who received real tDCS showed a significant improvement in their working memory. Furthermore, based on the results in Table 3 and Figure 2, participants in the real tDCS group had a 38.33% improvement in their working memory test scores, while the comparison group (tDCS Sham) showed only a 0.51% improvement. This difference was also statistically significant.
5. Discussion
This clinical trial aimed to investigate the effect of tDCS on the DLPFC in reducing cravings, decreasing depression, and improving working memory in cannabis users. The selection of this brain region was based on its crucial role in drug-seeking behaviors and cognitive functions (29).
Our study's statistical results demonstrate that the tDCS method was significantly effective in reducing cannabis cravings, decreasing depression, and improving working memory. There was a notable difference in the percentage of average changes between individuals who received active tDCS and those who received sham tDCS. However, it is worth noting that the sham tDCS group also showed some improvement, which could be attributed to the utilization of standard psychotherapeutic treatments, socio-environmental factors, and the natural progression of recovery leading to enhanced individual and social functioning.
The results of the current study are consistent with research conducted by Trojak et al., which demonstrated the effectiveness of transcranial electrical stimulation of the DLPFC in significantly reducing cravings and desire for alcohol (30). Additionally, the study conducted by Batista et al. provided further evidence for the positive effects of tDCS in reducing drug cravings in patients addicted to cocaine-crack (31).
Moreover, Wang et al. conducted a study that aligns with the findings of the current research. The study focused on the therapeutic application of tDCS in heroin-addicted patients, specifically targeting the bilateral fronto-lateral circuits with cathodes on T3 and T4 and anodes on O1 and O2, based on the 10 - 20 EEG system. Following the active treatment, the researchers reported a significant reduction in drug cravings (32).
In another study, Taremian et al. investigated the effects of tDCS on the DLPFC (right anode - left cathode) on the craving for opium in a group of opium users. The study results showed that active tDCS significantly reduced drug craving (33).
Martinotti et al. also examined the same protocol (right anode/left cathode) on participants with substance use disorder and gambling disorder. Their study reported a significant reduction in cravings in the intervention group (34). Similarly, Sharifi-Fardshad et al. conducted a study comparing the effects of active tDCS on the DLPFC using right anodal and left anodal protocols, along with a sham group. Their study revealed that active tDCS with right anodal stimulation was effective in reducing craving levels and cue-induced craving in crack-heroin users (35).
The effectiveness of brain electrical stimulation, specifically tDCS, in reducing cravings for methamphetamine use has been confirmed by studies conducted by Dadashi et al. Additionally, Moradi Kelardeh et al. reported that direct transcranial stimulation of the DLPFC resulted in a reduction in drug cravings and stress (36, 37).
However, contrary to the current study, the results of the study conducted by Garg et al. showed that the effect size of repeated tDCS sessions on the prefrontal cortex (left anode/right cathode) for reducing cravings in patients with moderate opioid addiction was modest, and it failed to produce a significant difference between the intervention and sham groups in terms of drug craving (38). Similarly, the results of the study conducted by Klauss et al. showed that active anodal tDCS did not significantly reduce the relapse rate of patients with opioid dependence enrolled in the MMT program (39).
In support of the findings, it can be stated that evidence suggests that the potential changes induced by transcranial electrical stimulation are likely due to the influence on neurotransmitter modulation in nerve cells (40).
Furthermore, these findings can be explained with reference to the incentive sensitization theory, which suggests that substance craving arises from the involvement of subcortical neural circuitry and the brain's reward system. Long-term changes in cortical excitability, specifically affecting dopamine neurotransmission, can explain the observed results. The heightened sensitivity of dopamine due to these underlying mechanisms leads to an increased salience of drug-related incentives (41).
The results of the present study align with the research conducted by Oraki et al., which demonstrated that tDCS effectively reduced the severity of depressive symptoms in individuals with depression disorders (42). Similarly, the study conducted by Vaghef et al. supports the significant reduction in depression scores observed in the experimental group compared to the control group. This reduction was noted after 10 sessions of tDCS, utilizing an intensity of 2 milliamperes (43).
Additionally, the results of the study conducted by Brunoni et al. indicated the effectiveness of tDCS in reducing depression (44). In a study by Narimani et al., the efficacy of tDCS in reducing substance craving, alleviating depression, and managing anxiety in university students was investigated. The findings indicated that tDCS was effective in reducing substance craving and alleviating symptoms of depression, although it did not significantly impact anxiety levels among the participants (45).
Csifcsák et al. (46) found that tDCS was effective in treating depression and reducing its symptoms. Furthermore, da Silva et al. reported that stimulation of the right or left prefrontal cortex led to a reduction in depression (16).
Treatment with tDCS over the skull enhances cognitive and behavioral performance and reduces dysfunctional processing in individuals with depression. This is because it engages the prefrontal cortex, which is involved in mood regulation and emotional processing. By increasing the brain's capacity for information processing and reducing maladaptive thoughts, tDCS leads to improved cognitive and behavioral functioning and decreased impaired processing in individuals with depression (47).
Drug addiction is associated with widespread structural and functional impairments in the prefrontal cortex, which manifest as extensive deficits in neurocognitive functions, particularly executive functions (16). In line with the findings of the present study, the results of the study conducted by Boggio et al. (2008) demonstrated that stimulation of the DLPFC is effective in enhancing working memory in healthy individuals (48).
The study by Boggio et al. showed that stimulation of the DLPFC improves visual recognition memory in individuals with Alzheimer's disease (48). Similarly, researchers have demonstrated that tDCS modulates working memory efficiency by altering brain activity, observing improved performance in working memory with anodal and cathodal tDCS treatment. These findings are consistent with the present study, confirming the beneficial effects of anodal and cathodal tDCS on working memory (49).
Furthermore, research conducted by Oraki et al. demonstrated that tDCS leads to improvements in working memory in individuals with depression disorders (42). The study by Shokreh and Hosseini also showed that electrical stimulation improves working memory in individuals with developmental coordination disorder (50).
Indeed, tDCS is a tool that enhances the brain's ability to process incoming information, and this characteristic enhances the effectiveness of other treatments. However, tDCS does not render individuals completely independent of other forms of treatment. It can be used as an adjunctive therapy to complement other interventions (51).
On the other hand, the findings of the current study contradict the results of the study by Mylius et al., which reported no improvement in working memory despite using a two-milliampere current (52). This discrepancy may be attributed to the one-week interval between the stimulation sessions. One hypothesis is that the observed effects are due to increased cortical excitability in the left DLPFC as a result of anodal stimulation, which depolarizes neurons and enhances their excitability in that region (50).
Based on the information provided above, we can conclude that tDCS can be effective in reducing cannabis cravings, improving depression levels, and enhancing working memory in cannabis users.
5.1. Limitations
When interpreting the findings of this study, it is important to consider the following limitations, despite the promising implications:
Firstly, we did not assess the duration of the therapeutic effects of the intervention on craving reduction. Future studies with long follow-up periods are recommended to address this aspect.
Secondly, another limitation of this study is the attrition rate, where some participants dropped out. However, it is worth noting that the participants who dropped out did not significantly differ from those who completed the study in terms of demographic and clinical characteristics.
Additionally, the NEUROSTIM-2 device conforms to the BS EN standard but lacks valid FDA and CE certifications at the next level.