1. Background
Substance use disorders (SUDs) are chronic and often recurring disorders that make individuals susceptible to serious problems such as AIDS, hepatitis, and other chronic conditions, ultimately leading to a reduced quality of life (QoL) (1). Additionally, this disorder is influenced by a combination of biological, social, psychological, and cultural factors (2, 3), and its high comorbidity with psychiatric disorders such as depression, anxiety, and personality disorders has severe effects on the QoL and the health of patients (4-7). Over time, individuals who use opioids may suffer from intense psychological distress and a diminished QoL (8). Dissatisfaction with life is more prevalent among opioid-dependent individuals compared to the general population (9).
Opioid substitution treatment (OST) is an evidence-based intervention for opioid dependence that improves patients' health and reduces mortality (10). One of the harm reduction treatment options is maintenance treatment with opioid agonists, where the patient discontinues the use of the illicit drug and instead uses medications like methadone, buprenorphine, and so on (11). Considering the psychological aspects of addiction, treatment plays a crucial role in reducing relapse, dropout rates, and increasing tolerance levels for abstinence, thereby improving the psychological symptoms that drive individuals towards substance use during treatment. Among the important factors in this regard is the focus on the QoL of individuals undergoing agonist maintenance treatment (12). Opioid replacement therapy improves the QoL in patients compared to those not receiving treatment (13). Since the majority of patients remain on OST indefinitely, it can be argued that improving the mental well-being and QoL of patients during OST should be a primary goal of patient care (14).
Over the past three decades, attention to QoL as a significant factor in evaluating treatment outcomes and treatment effectiveness in both physical and mental illnesses has increased (15). The World Health Organization (WHO) defines QoL as “an individual's perception of their position in life in the context of the culture and value system in which they live and in relation to their goals, expectations, standards, and concerns” (16).
The QoL is significantly compromised among substance users (17) and particularly among opioid-dependent individuals (18). It provides an empirical measure of how individuals function across various life domains and assesses the impact of treatment on the disease burden associated with SUDs (19). Broadly, there are two types of questionnaires for assessing QoL. Generic tools, such as the WHOQOL, Duke Health Profile, and various QoL measures, are designed for cross-population comparisons, while disease-specific tools focus on aspects relevant to particular populations, such as opioid-dependent individuals (20, 21).
The opioid substitution treatment quality-of-life (OSTQOL) Questionnaire, developed by Strada et al. at the Addiction Research Center of the University of Hamburg, Germany, in 2017, is the only instrument in addiction science that addresses specific QoL domains for patients undergoing opioid agonist treatment. This 38-item questionnaire covers personal development, mental distress, social contacts, material well-being, OST, and discrimination (14). This instrument, unique both nationally and internationally, provides researchers with a valuable and efficient tool to evaluate QoL in this population. Its lack of comparable counterparts highlights the critical need it fulfills, especially for researchers in the country aiming to understand and improve life quality among individuals undergoing opioid agonist treatment.
2. Objectives
Considering that there are approximately 7,029 substance abuse treatment clinics in Iran (22), there is no instrument in the Persian language to assess the QoL of patients undergoing agonist maintenance treatment during their treatment. This questionnaire will assist healthcare providers and policymakers in evaluating therapeutic interventions. Given the large number of patients receiving agonist treatment and the lack of information about their QoL during treatment, the need for an appropriate tool to assess the QoL in this group is evident. Therefore, this study was designed to investigate the psychometric properties of the Persian version of the OSTQOL Questionnaire.
3. Methods
3.1. Study Design
This study employed an analytical cross-sectional design. The study population consisted of all adult males undergoing OST who attended substance abuse treatment centers in the city of Rasht, Iran, in the year 2021. Eight substance abuse treatment centers from the five districts of the Rasht municipality were randomly selected. Approximately 50 individuals were then conveniently selected and included in the study from each center. After obtaining ethical approval from the Ethics Committee of Guilan University of Medical Sciences (ethical code IR.GUMS.REC.1399.495) and obtaining informed consent from the patients, the current research was conducted.
The inclusion criteria for the study were receiving agonist treatment and a willingness to participate in the research. The exclusion criteria included the presence of serious psychiatric illnesses or chronic medical illnesses based on the individual's self-report and clinical interviews conducted by the study's first author. Considering that ten participants are required for each questionnaire item (23), and our study instrument contains 38 items, the sample size for this study was determined to be 380 individuals (24).
The data were analyzed using SPSS version 22 and LISREL software version 8.80. Since confirmatory factor analysis (CFA) in this study was conducted using the LISREL software, which does not perform the analysis in the presence of missing data, a double-check was performed both during the questionnaire collection phase and the data entry phase.
3.2. Translation
In this study, after obtaining permission from the original tool's developer and making the necessary coordination, the OSTQOL Questionnaire was translated through forward and backward translation methods by experts proficient in both the Persian and English languages. Initially, the main questionnaire was translated from the original language to Persian by two translators familiar with both Persian and English. Then, the two initial translations were combined and transformed into a single translation. In the next step, the back-translation of the final translated version from Persian to the original language was performed by a bilingual translator proficient in both Persian and English. The final translated English version was compared to the original version.
3.3. Content Validity
To assess the validity, a panel of experts was formed. This panel included three psychiatrists, a Ph.D. in statistics, two psychologists (one holds a Master's degree in Clinical Psychology with 15 years of experience in a psychiatric hospital, and the other has a Ph.D. in Psychology with over 20 years of therapeutic experience), two Ph.D. holders in addiction studies, and a general practitioner with experience in addiction treatment. This group evaluated the translated questionnaire items based on relevance, clarity, and simplicity, and assigned scores accordingly. In this manner, the questionnaire was assessed for grammar, sentence structure, and specificity. Each expert expressed their opinion on each item using a Likert scale ranging from irrelevant, unclear, and not fluent to completely relevant, clear, and fluent in terms of relevance, clarity, and simplicity of the tool. Ultimately, the Content Validity Index (CVI) was determined. A value equal to or higher than 0.80 was considered acceptable (25). For content validity ratio (CVR), the group expressed their opinions on the necessity of each item on a three-point Likert scale. There were 9 experts, and based on the Lawshe table, items with a CVR equal to or greater than 0.78 were deemed acceptable (26).
3.4. Face Validity
A group of twenty study participants with different age conditions and educational levels were selected to evaluate the face validity of the questionnaire items. Each item was read aloud to each participant, and their comments regarding the clarity of the questionnaire's questions, difficulty in understanding them, and relevance to the questionnaire's purpose were noted. Ultimately, their feedback was incorporated into the questionnaire.
3.5. Structural Validity
To determine the structural validity of the Persian version of the QoL Questionnaire in patients receiving OSTQOL, CFA was performed using LISREL 8.80 software.
3.6. Reliability
Cronbach's alpha and McDonald’s Omega coefficients were used to assess the reliability of the questionnaire. For each subscale of the questionnaire, Cronbach's alpha and McDonald’s Omega coefficients were calculated separately, and values above 0.70 were considered acceptable (27). To evaluate the stability of the questionnaire, the test-retest method with the measurement of the intraclass correlation coefficient (ICC) was used. Thirty participants in the OSTQOL Questionnaire completed it again after a 15-day interval. An ICC of 0.40 and above is considered acceptable (23).
3.7. Instrument
3.7.1. The Opioid Substitution Treatment Quality of Life Questionnaire
The OSTQOL Questionnaire was developed by Strada et al. in 2017. The initial version of this questionnaire consisted of 82 items, and the final version contains 38 items, comprising 6 subscales: Personal development (10 items), mental distress (9 items), social contacts (6 items), material well-being (3 items), OST (6 items), and discrimination (4 items). The scoring for this scale is based on a 5-point Likert scale (0: Does not apply to me at all, 1: Applies to me to some extent, 2: Applies to me to a moderate extent, 3: Applies to me to a considerable extent, 4: Applies to me to a great extent). According to the results reported by the developers of this questionnaire, the internal consistency reliability of its subscales was acceptable, with Cronbach's alpha ranging from 0.75 to 0.88. Additionally, the instrument demonstrated good and acceptable discriminant validity and convergent validity (14).
3.7.2. The Researcher-Made Questionnaire
The Researcher-Made Questionnaire includes demographic characteristics of participants and substance use profiles.
4. Results
This study was conducted on 380 adult males receiving OST. The participants' mean age was 44.2 ± 11.3 years. Among the study sample, 74.7% were undergoing methadone treatment, 18.2% were receiving tincture of opium treatment, and 6.8% were undergoing treatment with buprenorphine-naloxone, a partial agonist.
4.1. Content Validity
The CVR was calculated for all items of the questionnaire, and all values were greater than 0.78, indicating acceptability. Additionally, the CVR Scale was calculated to be 0.94, which is acceptable. The CVI revealed that items 1, 8, 9, 10, 29, and 34 had scores less than 0.80 for simplicity before modification, but after receiving feedback from experts in the second expert panel session, these scores were improved to a range of 0.90 to 1. Furthermore, items 9, 10, and 29 had scores less than 0.80 for relevancy but were enhanced to a score of 1 after expert panel feedback. Lastly, items 9, 10, 14, 15, 29, and 34 had scores less than 0.80 for clarity but were improved to a score of 1 after expert panel feedback. Content Validity Index-Scale, after revising items for simplicity, relevancy, and clarity, ranged between 0.89 and 0.95, which is above 0.8 and therefore acceptable and satisfactory.
4.2. Structural Validity
To examine the factor structure of the QoL Questionnaire in patients receiving OST, a CFA was conducted. In order to refine the model, items with t-values between 1.96 and -1.96 should be removed from the model. Based on the results obtained, none of the items were removed from the model. The results of model fit indices for the QoL Questionnaire in patients receiving OST are presented in Table 1. According to the results, the six-factor model of the questionnaire in the study group demonstrates an acceptable fit. In other words, the fit indices indicate that the collected empirical data support the theoretical model.
| Index | Persian Equivalent | Specified Model | Acceptable Fit Range |
|---|---|---|---|
| CMIN/DF | Standardized chi-square | 2.34 | < 3 |
| GFI | Goodness of Fit Index | 0.82 | > 0.90 |
| AGFI | Adjusted Goodness of Fit Index | 0.8 | > 0.90 |
| RMR | Root mean square residual | 0.087 | < 0.05 |
| RMSEA | Root mean square error of approximation | 0.06 | < 0.05 |
| NFI | Normed Fit Index | 0.95 | > 0.90 |
| CFI | Comparative Fit Index | 0.97 | > 0.90 |
| IFI | Incremental Fit Index | 0.97 | > 0.90 |
| PNFI | Parsimonious Normed Fit Index | 0.88 | > 0.50 |
The CFA was used to examine the factor structure of the QoL Questionnaire in patients receiving opioid maintenance treatment. To modify the model, questions with t-values between -1.96 and +1.96 had to be removed. The findings in Table 2 indicate that none of the questions were removed from the model.
| Items | Factor Loading | t | R2 |
|---|---|---|---|
| 1 | 81.0 | 12.18 | 66.0 |
| 2 | 83.0 | 85.24 | 70.0 |
| 3 | 84.0 | 28.19 | 70.0 |
| 4 | 83.0 | 23.19 | 69.0 |
| 5 | 83.0 | 24.19 | 69.0 |
| 6 | 81.0 | 46.18 | 65.0 |
| 7 | 66.0 | 13.14 | 44.0 |
| 8 | 79.0 | 67.17 | 62.0 |
| 9 | 73.0 | 15.16 | 54.0 |
| 10 | 59.0 | 21.12 | 35.0 |
| 11 | 76.0 | 23.15 | 57.0 |
| 12 | 78.0 | 65.21 | 61.0 |
| 13 | 80.0 | 98.15 | 64.0 |
| 14 | 83.0 | 63.16 | 69.0 |
| 15 | 81.0 | 17.16 | 65.0 |
| 16 | 68.0 | 26.13 | 46.0 |
| 17 | 70.0 | 69.13 | 49.0 |
| 18 | 75.0 | 88.14 | 56.0 |
| 19 | 66.0 | 95.12 | 44.0 |
| 20 | 82.0 | 78.16 | 67.0 |
| 21 | 76.0 | 95.16 | 59.0 |
| 22 | 87.0 | 53.20 | 76.0 |
| 23 | 79.0 | 67.17 | 62.0 |
| 24 | 85.0 | 58.19 | 72.0 |
| 25 | 82.0 | 42.16 | 68.0 |
| 26 | 84.0 | 82.16 | 70.0 |
| 27 | 90.0 | 13.18 | 81.0 |
| 28 | 43.0 | 29.8 | 19.0 |
| 29 | 82.0 | 14.22 | 67.0 |
| 30 | 82.0 | 14.22 | 67.0 |
| 31 | 79.0 | 39.17 | 63.0 |
| 32 | 87.0 | 47.19 | 75.0 |
| 33 | 81.0 | 81.17 | 66.0 |
| 34 | 83.0 | 52.18 | 69.0 |
| 35 | 36.0 | 69.5 | 13.0 |
| 36 | 94.0 | 29.5 | 89.0 |
| 37 | 60.0 | 34.6 | 36.0 |
| 38 | 55.0 | 13.6 | 30.0 |
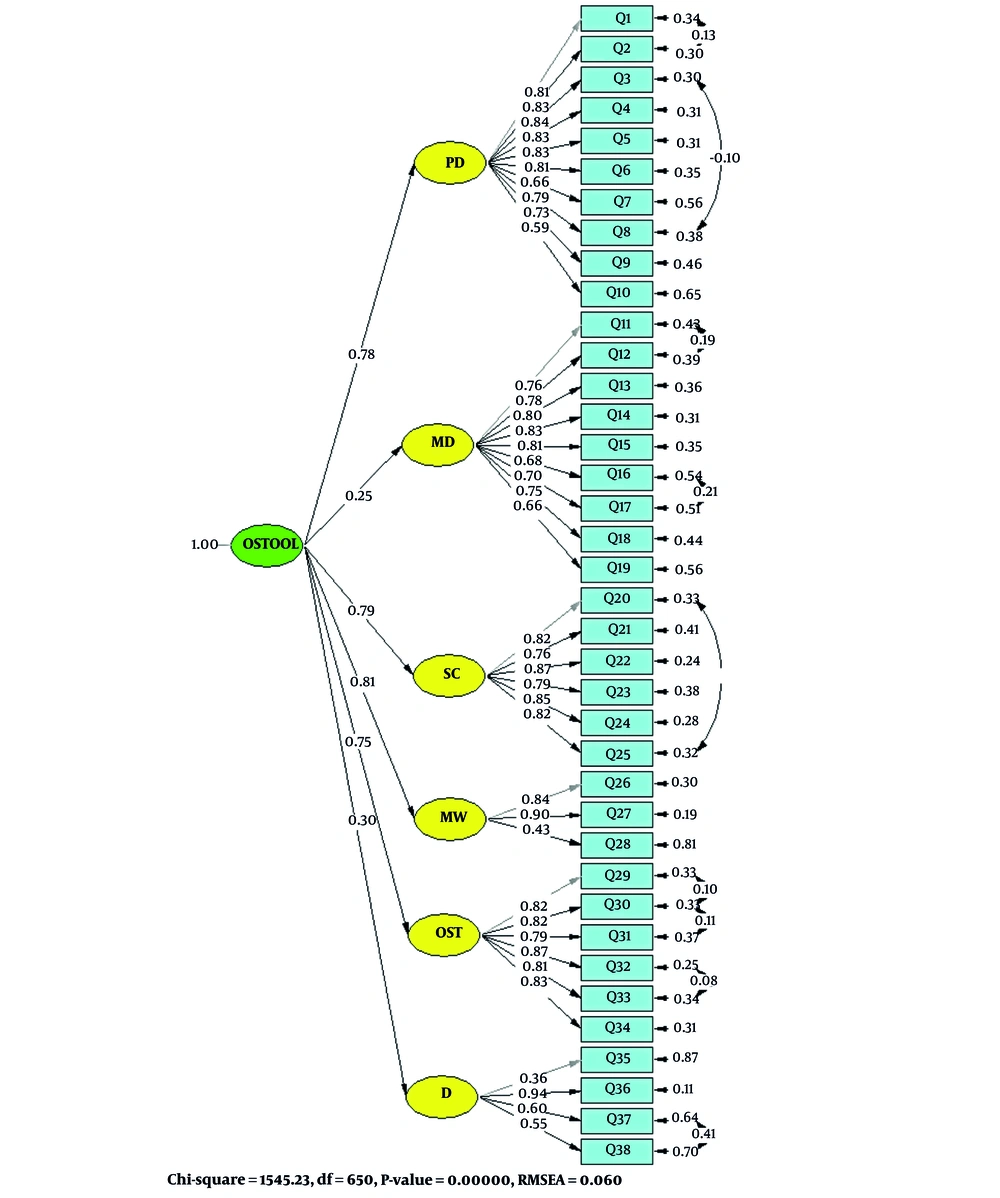
The measurement model for the items of the QoL Questionnaire in patients receiving OST was presented using a standard CFA model, as shown in Figure 1.
Measurement model of the Quality-of-Life (QoL) Questionnaire in patients receiving opioid substitution treatment (OST) based on the six-factor model, in standard estimation using confirmatory factor analysis (CFA); PD, personal development; MD, mental distress; SC, social contacts; MW, material wellbeing; D, discrimination.
4.3. Reliability
The internal consistency of the QoL Questionnaire in patients receiving OST was assessed using Cronbach's alpha coefficient and McDonald’s Omega Coefficient, resulting in values of 0.926 and 0.918, respectively, indicating excellent stability. Then, Cronbach's alpha and McDonald’s Omega Coefficients were calculated for each of the factors, and the coefficients obtained demonstrated adequate stability in all domains (Table 3).
| Dimensions | Cronbach's Alpha Coefficient | McDonald’s Omega Coefficient |
|---|---|---|
| Personal development | 0.935 | 0.935 |
| Mental distress | 0.924 | 0.924 |
| Social contacts | 0.923 | 0.923 |
| Material well-being | 0.612 | 0.618 |
| OST | 0.932 | 0.932 |
| Discrimination | 0.684 | 0.680 |
| Total | 0.926 | 0.918 |
Abbreviation: OST, opioid substitution treatment.
To determine the questionnaire's stability in terms of reproducibility, the ICC and test-retest reliability coefficient were used. The findings indicated that the questionnaire demonstrated excellent stability. The ICC value for the questionnaire was 0.943, which was statistically significant (P < 0.001). Additionally, the test-retest reliability coefficient for the research sample was 0.996 (Table 4).
| Questionnaire | Test-Retest Coefficient | ICC | 95% Confidence Interval for ICC | P-Value | |
|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||
| QoL | 0.996 | 0.943 | 0.772 | 0.986 | < 0.001 |
Abbreviation: ICC, intraclass correlation coefficient; QoL, quality of life.
5. Discussion
This study aimed to systematically translate the OSTQOL Questionnaire into Persian and assess the validity and reliability of the translated version. The questionnaire was translated using the standard forward-backward translation method (28), and the results obtained in the content validity phase indicated that the tool had good content validity and was well understood by both expert groups and patients receiving agonist treatment.
To investigate the factor structure of the QoL Questionnaire in patients receiving OST, CFA was employed. Fit indices such as χ2/df, GFI, AGFI, RMSEA, NFI, CFI, and IFI indicated that all of them were acceptable for the instrument. Despite the RMR value (0.087) exceeding the ideal < 0.05 threshold, all key global fit indices (e.g., CFI, RMSEA, NFI, CFI, GFI) indicate acceptable model fit. Since item variances are consistent and other indices do not suggest misfit, the elevated RMR likely reflects minor residual discrepancies or model complexity, rather than serious model misspecification.
Cronbach's alpha and McDonald’s Omega Coefficients were used to assess internal consistency for the QoL Questionnaire in patients receiving OST, and the results indicated that the coefficients were close to or greater than 0.70 (0.61 - 0.93) for both the overall instrument and all its subscales, demonstrating desirable internal consistency. The resemblance between apha and Omega indicates that the assumption of tau-equivalence (equal contributions of items to the latent construct) is likely met. In other words, all items in the scale or subscales have similar factor loadings on the latent variable (29, 30), and Cronbach’s alpha is a valid estimate of reliability in this case.
In Strada et al.'s study (14), Cronbach's alpha coefficients ranged between 0.75 and 0.88, with the lowest value attributed to the material well-being factor. Similarly, in the present study, this factor also exhibited the lowest Cronbach's alpha coefficient. To determine the questionnaire's stability in terms of reproducibility, the ICC and test-retest reliability were employed. The findings demonstrated that the questionnaire exhibited excellent stability.
This study is, to date, the only one that has translated this tool into Persian while also evaluating its validity and reliability. Therefore, no similar example is found in the scientific literature, making comparisons with similar studies unfeasible. However, some research in the field of addiction has utilized this tool to examine primary outcomes, and several studies currently underway employ it as a fundamental and core instrument (31-34).
However, it is worth noting a limitation of this study. All participants in this study were male, which was partly due to the limited number of treatment-seeking women for SUD due to social stigma. Additionally, since this study was conducted during the COVID-19 pandemic, it was associated with more restrictions on patient referrals. As a result, the generalizability of the questionnaire’s psychometric properties to female populations is limited. Future studies should validate the instrument among females to ensure its applicability across sexes.
5.1. Conclusions
Based on the findings of the current research, the OSTQOL Questionnaire demonstrates acceptable validity and reliability for individuals undergoing OST. This instrument serves as an accessible and user-friendly tool for completion in questionnaire format, utilizing the language of the patients. It transforms it into a promising tool not only for research purposes but also for routine patient care. The OSTQOL can be employed for monitoring the QoL of patients and evaluating new interventions. Furthermore, it can inform researchers, healthcare providers, and policymakers about the needs of patients, serving as motivation for the development of novel interventions to enhance the QoL.