1. Background
Opioid addiction has become a significant public health and social issue in Iran over the past two decades (1). According to reports from the United Nations Office on Drugs and Crime, Iran has one of the highest rates of addiction worldwide (2). Recent estimates suggest that there are 1 - 2 million opioid-dependent individuals in Iran (3). The serious economic, political, cultural, and health-related consequences of opioid abuse in society demand increased attention to treatment and control methods.
One therapeutic approach is methadone maintenance therapy. Methadone is a synthetic opioid agonist with a higher affinity for opioid receptors than heroin and its derivatives (4). It is administered orally and alleviates withdrawal symptoms in opioid-dependent patients. The duration of methadone use can vary from short-term (7 - 30 days) to long-term (up to 180 days) and maintenance treatment (beyond 180 days). Studies have shown the advantages of methadone use, including a reduction in the risk of opioid-related mortality by approximately 70%, a decrease in crime rates, a lower risk of HIV and hepatitis C and B in injection opioid users, an improved quality of life, and reduced opioid use (5). However, methadone use causes unintended side effects. Studies have indicated that methadone can cause side effects such as drowsiness, nausea, vomiting, constipation, weight gain, insomnia, depression, seizures, and sexual dysfunction in men (6-8). Sexual dysfunction in men resulting from methadone use significantly affects their quality of life. It may lead to a reduced willingness to continue methadone treatment, thereby interfering with its known therapeutic benefits. Studies have shown that individuals undergoing methadone treatment experience sexual dysfunction, with 42% reporting difficulties in erectile function. This condition disrupts marital relationships and increases the likelihood of relapse (9). One study demonstrated that 60.5% of individuals undergoing methadone treatment experienced erectile dysfunction, and 70.7% reported reduced sexual desire (10).
Methadone-induced sexual disorders are common and problematic for individuals under methadone treatment (11). Methadone has disruptive effects on the hypothalamic-pituitary-gonadal (HPG) axis and sexual function (12). Opium addiction causes hypogonadism, decreased libido, erectile dysfunction, and infertility. Additionally, methadone-treated patients have lower levels of blood testosterone and higher levels of prolactin due to inhibited LH (luteinizing hormone) secretion (13). Few pharmaceutical therapies have proven effective, such as testosterone replacement therapy, PDE5 inhibitors, bupropion, trazodone, opioid antagonists, and plant-derived medicines like Rosa damascena and ginseng (14). Non-pharmacological options, such as psychosexual or physical therapies, should also be considered (15).
Bupropion is a dopamine reuptake inhibitor used to treat depression. It is a one-ring amino ketone compound with a structure similar to amphetamines (16). It is well absorbed through the gastrointestinal system and is often added to selective serotonin reuptake inhibitors (SSRIs) to counteract sexual side effects. Studies have suggested that bupropion may enhance sexual arousal, orgasm, and satisfaction (17).
Amantadine is a dopamine agonist that likely enhances sexual responses by stimulating the dopaminergic pathways. Studies have shown that its continuous use can increase sexual responses without tolerance, even in rodents (18).
Considering that sexual dysfunction in patients undergoing methadone treatment can lead to reduced compliance and consequently a decrease in therapeutic effects, we decided to address the lack of research in this area. Therefore, we conducted a study on male patients from the North Iranian population.
2. Objectives
This study aimed to compare the impact of bupropion and amantadine on sexual function in methadone-dependent males.
3. Methods
The present study was a clinical trial conducted following approval from the Research Council of Babol University of Medical Sciences and the University's Ethics Committee, with an ethics code of MUBABOL.HRI.REC.1396.108. The study was conducted in February 2019. The study population consisted of all methadone-dependent male patients seeking treatment at the Addiction Treatment Center of Shahid Zakerian in Babol. The indication for methadone treatment was a history of opium and heroin use disorder.
The sample size was determined using a formula, considering a type I error rate of 5%, a standard deviation of 1.1 for sexual function scores in both groups, and an effect size of 0.9. The minimum required sample size for each group was 32 individuals. Convenience sampling was used for sample selection. Patients were randomly assigned to two groups using the block randomization method. A block size of 4 was chosen, possible combinations of amantadine and bupropion were calculated, blocks were randomly selected to determine the assignment of all participants, and written consent was obtained (19). The study was double-blind.
Inclusion criteria were patients aged 20 to 70 years with a history of opioid dependence, seeking addiction treatment at the affiliated addiction treatment centers, and receiving methadone maintenance therapy. Exclusion criteria included female patients, unmarried male patients, patients who did not consent to participate in the study, patients with medical conditions affecting sexual function (e.g., diabetes, known vascular and endocrine disorders, and known neurological disorders affecting sexual function), patients taking any medication known to affect sexual function (e.g., amphetamines), and patients with psychological disorders.
The data collection tools included a demographic information questionnaire specific to each patient and a checklist of medication side effects. These recorded the patient’s age, weight, height, body mass index, duration of addiction, duration of abstinence, number of withdrawal attempts, and treatment-related side effects.
The International Index of Erectile Function (IIEF) Questionnaire consists of 15 questions assessing five domains of sexual function: Erectile function (6 questions), sexual desire (2 questions), orgasmic function (2 questions), intercourse satisfaction (3 questions), and overall satisfaction (2 questions). Higher scores indicate better sexual function, with a maximum score of 75. The severity of sexual dysfunction was classified based on the obtained scores as follows: 0 - 15 (severe), 16 - 31 (moderate), 32 - 47 (mild to moderate), 48 - 63 (mild), and 64 - 75 (no dysfunction). Patients with sexual dysfunction based on this questionnaire were included in the study, whereas those without sexual dysfunction were excluded. Patients were randomly allocated to two groups: One receiving bupropion and the other receiving amantadine. After one month, all patients completed the IIEF questionnaire again, and their scores were compared to their initial scores (20).
The General Health Questionnaire (28-GHQ) was used to assess confounding factors, such as depression and other psychological issues that may affect sexual function. The 28-GHQ was completed by individuals before the intervention (medication administration) to identify those with psychological disorders so they could be excluded from the study. Psychological disorders can significantly affect sexual functions. The 28-GHQ is a standard, valid, and reliable screening questionnaire based on self-reporting. It is widely used in clinical settings to screen individuals for psychological disorders (21). The original version of this questionnaire contains 60 questions, but 30-, 12-, and 28-item versions also exist (22). The 28-item version has been extensively used to assess mild-to-severe psychological disorders in various situations, with reliability and validity estimated at 93% and 91%, respectively (23). In this study, the 28-item version of the GHQ was used, and scoring was based on the Likert method, where each answer was scored as 0 (better than usual), 1 (same as usual), 2 (less than normal), or 3 (much less than normal). Scores for each participant in each subscale were calculated separately. The scores of the four subscales were then added to obtain a total score, which was used to assess the participants’ psychological status. The lowest possible score represents the best psychological health, while the highest score indicates the poorest psychological health. This questionnaire has five scoring methods, with the Likert scoring method being the best, and a maximum score of 84. The cutoff point for this questionnaire has been determined in various studies in the country to be between 21 and 23. A 23-point cutoff was applied to indicate overall psychological dysfunction, with a 14-point cutoff for the subdomains (24). The 28-GHQ was administered to all patients to identify individuals with psychological disorders who were excluded from the study. Patients were randomly allocated to two groups: One group received amantadine (100 mg twice daily, Abidi), and the other group received bupropion (150 mg once daily at 4 PM, Abidi). After one month, all patients completed the IIEF Questionnaire again, and their scores were compared to their initial scores. The reliability and validity of the questionnaire in the Persian language were confirmed (25).
After collecting the data from the checklist, we analyzed the data using descriptive and inferential statistics with SPSS-24 software. Independent sample t-tests, paired sample t-tests, and chi-squared tests were used to compare and assess the relationships between the two groups under various conditions.
4. Results
The study was conducted to compare the effects of bupropion and amantadine on reducing sexual dysfunction in 47 methadone-dependent males. Of these, 23 patients were in the amantadine group, and 24 were in the bupropion group. All patients were married; 54.3% (25 individuals) had less than a diploma, 32.6% (15 individuals) had a diploma, and 13% (6 individuals) had education beyond a diploma. The demographic data are presented in Table 1.
The mean and standard deviation of the duration of methadone use in the amantadine group were 1.74 ± 1.48 years, with a minimum of 1 year and a maximum of 6 years. In the bupropion group, these values were 2.3 ± 3.39 years, with a minimum of 1 year and a maximum of 15 years (Table 1).
The distribution of the studied patients based on the type of medication used in the two groups showed that nearly 89.5% (34 patients) used opium, while the remaining 11.6% (4 patients) used heroin. The distribution of patients based on the duration of methadone use showed that 36.2% (17 people) used methadone for less than one year, 10.6% (5 people) used it for 5 - 10 years, and 21.3% (10 people) used it for more than 10 years (Table 1).
The mean, minimum, and maximum doses of methadone in the two study groups were similar. Independent sample t-tests revealed that the two groups did not have statistically significant differences in demographic variables, including age, duration of addiction, duration of methadone use, and methadone dosage, with P-values greater than 0.05. The chi-square test also indicated that the two groups had similar education levels (P > 0.05) (Table 1).
Assessment of general health status as an entry criterion for the study revealed no statistically significant difference between the two groups in the overall general health score and its various dimensions, including physical health, anxiety, social function, and depression (P > 0.05). None of the patients in either group had a score of more than 23, indicating good mental health.
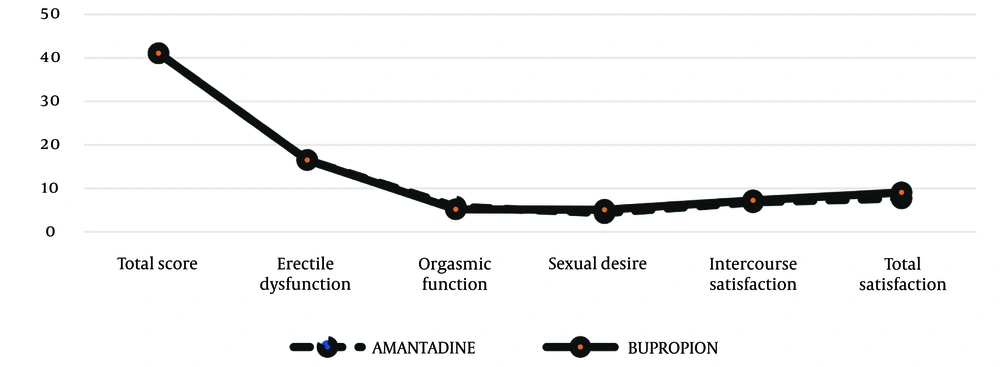
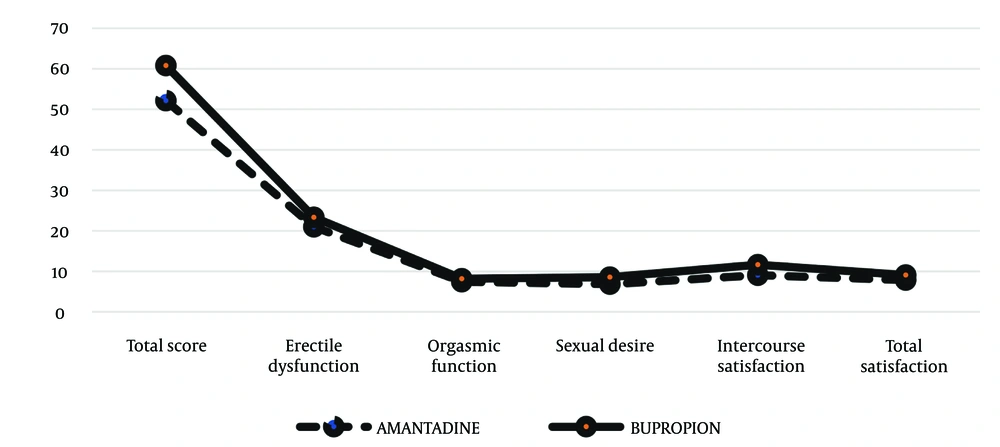
Independent sample t-tests were performed to compare the mean scores for sexual dysfunction, erectile dysfunction, orgasm function, sexual desire, satisfaction with intercourse, and overall satisfaction before the intervention in the two study groups. As illustrated in Figure 1, no statistically significant differences were found (P > 0.05) (Table 2). Independent sample t-tests were also conducted to compare the mean scores of sexual dysfunction and its various dimensions after the intervention between the two study groups. The results in Table 3 showed a statistically significant difference between the two groups in the total sexual dysfunction score (52.13 ± 14.07 vs. 60.79 ± 4.47, P = 0.006) (Figure 2). Additionally, the bupropion group had higher scores in sexual desire (6.83 ± 2.42 vs. 8.58 ± 1.18, P = 0.003), satisfaction with intercourse (9.04 ± 3.15 vs. 11.62 ± 1.44, P = 0.001), and overall satisfaction (7.83 ± 2.65 vs. 9.08 ± 0.93, P = 0.034) compared to the amantadine group.
| Variables | Amantadine | Bupropion | P-Value |
|---|---|---|---|
| Total score | 41.08 ± 15.57 | 41.08 ± 11.43 | 0.999 |
| Erectile dysfunction | 16.52 ± 8.15 | 16.54 ± 5.17 | 0.992 |
| Orgasmic function | 5.78 ± 3.13 | 5.17 ± 2.82 | 0.482 |
| Sexual desire | 4.30 ± 1.46 | 5.08 ± 1.64 | 0.093 |
| Intercourse satisfaction | 6.78 ± 3.70 | 7.25 ± 3.07 | 0.639 |
| Total satisfaction | 7.69 ± 2.42 | 7.04 ± 2.14 | 0.331 |
Comparison of the Two Groups Before the Intervention a
| Variables | Amantadine | Bupropion | P-Value |
|---|---|---|---|
| Total score | 52.13 ± 14.07 | 60.79 ± 4.47 | 0.006 |
| Erectile dysfunction | 21 ± 5.75 | 23.33 ± 2.54 | 0.077 |
| Orgasmic function | 7.43 ± 2.29 | 8.17 ± 1.20 | 0.175 |
| Sexual desire | 6.83 ± 2.42 | 8.58 ± 1.18 | 0.003 |
| Intercourse satisfaction | 9.04 ± 3.15 | 11.62 ± 1.44 | 0.001 |
| Total satisfaction | 7.83 ± 2.65 | 9.08 ± 0.93 | 0.034 |
Comparison of the Two Groups After the Intervention a
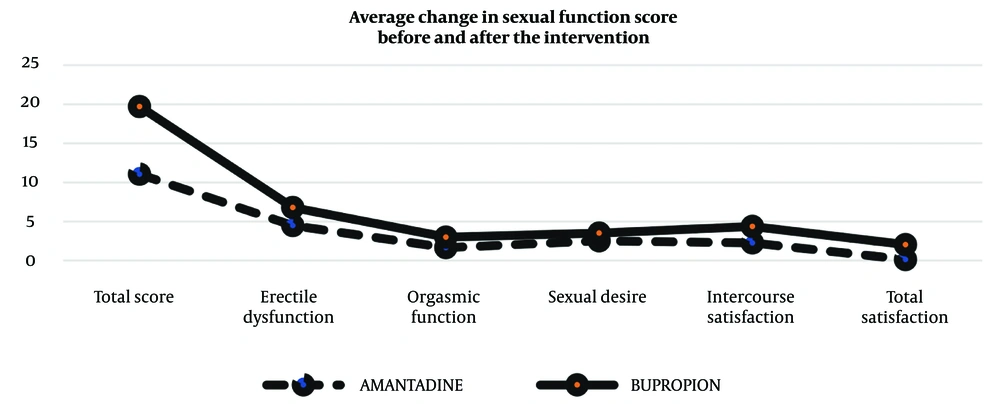
A comparison of the mean changes in sexual dysfunction scores and their various dimensions before and after the intervention, using an independent t-test, showed a statistically significant difference between the two groups in terms of changes in the total sexual dysfunction score (11.04 ± 9.18 vs. 19.71 ± 10.89, P = 0.005) (Table 4). Additionally, the bupropion group had greater changes in satisfaction with intercourse (2.26 ± 2.42 vs. 4.37 ± 3.09, P = 0.012) and overall satisfaction (0.13 ± 1.68 vs. 2.04 ± 2.07, P = 0.001) compared to the amantadine group (illustrated in Figure 3).
| Variables | Amantadine | Bupropion | P-Value |
|---|---|---|---|
| Total score | 11.04 ± 9.18 | 19.71 ± 10.89 | 0.005 |
| Erectile dysfunction | 4.48 ± 5.19 | 6.79 ± 5.26 | 0.136 |
| Orgasmic function | 1.65 ± 1.80 | 3 ± 3.20 | 0.084 |
| Sexual desire | 2.52 ± 2.46 | 3.5 ± 2.08 | 0.148 |
| Intercourse satisfaction | 2.26 ± 2.42 | 4.37 ± 3.09 | 0.012 |
| Total satisfaction | 0.13 ± 1.68 | 2.04 ± 2.07 | 0.001 |
Comparison of the Average Changes Before and After the Intervention a
Pearson's correlation test showed a significant correlation between the mean changes in sexual dysfunction score and its dimensions and the amount of methadone used in the two study groups. Although the correlation was negative—meaning that an increase in methadone use was associated with a decrease in the sexual dysfunction score—this correlation was not statistically significant in either of the study groups (P > 0.05). According to the results, the greatest difference was observed in changes in satisfaction with intercourse and overall satisfaction.
The comparison of side effects resulting from medication use showed no statistically significant difference between the two groups: 60.9% in the amantadine group and 54.2% in the bupropion group. However, it is worth noting that the occurrence of adverse events was lower in the bupropion group compared to the amantadine group.
5. Discussion
The current study investigated sexual dysfunction in 47 methadone-dependent male patients using the IIEF questionnaire and evaluated the effects of bupropion and amantadine on enhancing sexual function. The results of the statistical tests conducted before the intervention indicated that randomization among the patients was adequate. Demographic variables, including age, duration of addiction, methadone dosage, education level, type of opioid used, and history of physical illnesses, showed no statistically significant differences between the two study groups prior to the intervention. Additionally, both groups had similar scores on the 28-GHQ, indicating comparable levels of mental health before the intervention. There were no significant differences in scores for sexual dysfunction, erectile dysfunction, orgasmic function, sexual desire, sexual satisfaction, or overall satisfaction between the two groups before the intervention.
However, the results demonstrated a significant difference between the two groups after treatment. The total sexual dysfunction scores improved significantly more in the bupropion group compared to the amantadine group. Furthermore, significant differences were observed between the two groups in terms of sexual desire, sexual satisfaction, and overall satisfaction, with higher scores in the bupropion group. This indicates that bupropion has a more favorable impact on sexual function.
The comparison of the severity of sexual dysfunction before the intervention in the two study groups—amantadine and bupropion—using the chi-square test, revealed that in the amantadine group, the severity of sexual dysfunction was severe and moderate, accounting for 13.04% and 17.39%, respectively. In the bupropion group, these percentages were 0% for severe and 29.17% for moderate, with no significant difference between the two groups. However, after the intervention, the severity of sexual dysfunction in the amantadine group was 8.7% for severe and 13.04% for moderate, while it was 0% in the bupropion group, indicating a significant difference. In other words, bupropion led to an improvement in the severity of patients' sexual dysfunction.
The comparison of adverse effects resulting from medication use, using the chi-square test, showed that adverse effects in the amantadine group were 60.9%, compared to 54.2% in the bupropion group. This indicates that adverse effects were less common in the bupropion group.
Yee et al.'s study supported the positive effect of bupropion in increasing sexual satisfaction among methadone users, which is consistent with the results of the current study (26).
Safarinejad conducted a study investigating the effects of bupropion as an adjunct therapy for sexual dysfunction caused by SSRIs in men. They found that bupropion had a significant positive impact on sexual function, including sexual desire, orgasm, and satisfaction, which was also observed in this study (27).
Studies by Tatari et al. and a review by Pereira et al. have also supported the beneficial effects of bupropion in treating sexual dysfunction, particularly those caused by methadone use, which was confirmed in this study (28). These studies suggest that bupropion not only acts as an effective antidepressant but also has fewer adverse effects on sexual function, potentially improving sexual function in certain individuals (29).
Several studies have consistently demonstrated the positive effects of bupropion on sexual function and satisfaction in different populations, further supporting its potential as a treatment option for sexual dysfunction (30). The results of this study indicate that bupropion has a positive effect on reducing sexual dysfunction in individuals who consume methadone compared with amantadine, which is confirmed by previous studies. This positive effect of bupropion on sexual function includes increased sexual desire, sexual satisfaction, and overall sexual function. Furthermore, it suggests positive changes in the severity of sexual dysfunction and its various dimensions after intervention with bupropion.
This study is consistent with previous research on the impact of bupropion on sexuality and sexual satisfaction, confirming that bupropion may lead to significant improvements in sexual dysfunction in individuals who use methadone. The study may provide valuable insights for healthcare professionals and specialists in improving sexual function in individuals who use methadone and in selecting more suitable treatment options. This is supported by the better sexual function scores observed in patients treated with bupropion compared to those treated with amantadine. Additionally, the occurrence of adverse effects was less frequent in the bupropion group compared to the amantadine group. These findings collectively indicate the potential of bupropion as a treatment for sexual dysfunction in individuals using methadone and suggest that further research should explore the impact of different medication doses on patients' sexual function.
5.1. Conclusions
In conclusion, given the negative impact of methadone on sexual function in patients at addiction treatment clinics, it is advisable to screen patients for sexual dysfunction before initiating any treatment. This proactive approach can help prevent the worsening of sexual dysfunction and improve the quality of intimate relationships. This study highlights the positive impact of bupropion on sexual function and satisfaction among methadone users, suggesting that bupropion may be a more effective choice than amantadine for improving sexual function in this population.
5.2. Limitation and Suggestion
Factors contributing to the reduction in sample size included immigration, unwillingness to continue treatment, non-acceptance of medication, and changes in doctors. Further studies with larger sample sizes are needed to confirm the findings of this study.