1. Background
In December 2019, a new coronavirus, called COVID-19, caused a global pandemic, leading to over 6.5 million deaths (1). COVID-19 is commonly manifested by fever, cough, respiratory distress, fatigue, myalgia, headache, vertigo, constipation, and heartburn (2-5).
Sexual dysfunction is a condition in which individuals are not satisfied with sexual activity during the sexual relationship. In addition to organic causes of sexual dysfunction (e.g., vascular, hormonal, neurogenic, and pharmacological causes), psychogenic reasons (e.g., depression and anxiety) can also negatively affect sexual activity in women and men (6). Anxiety and depression resulting from the COVID-19 outbreak (7) were possible causes for putting couples' relationships at risk, creating a chaotic environment that leads to psychological effects in couples.
Previous studies have reported negative impacts of COVID-19 on men's sexual performance. For example, the risk of developing erectile dysfunction in COVID-19 patients was estimated to be 2.64 times that of the control group (8). In a case report, two anorgasmia cases were documented as complications after recovery from COVID-19 (9). In another report, the prevalence of erectile dysfunction was reported as 64.7% using the International Index of Erectile Function (IIEF) questionnaire in 153 COVID-19 patients, and the severity was mostly mild (10). In a follow-up study, the authors reported a significant reduction to 50.3% after three months (11).
Reviewing the literature, although the extra-pulmonary manifestations of COVID-19 have been studied extensively, there are limited studies on the pathophysiological involvement of COVID-19 in the male reproductive system. More importantly, the long-term effects of the disease on sexual function appear to be neglected.
2. Objectives
In this study, we aimed to evaluate short and long-term male sexual function as a function of being infected with COVID-19.
3. Methods
During February-March 2022, one hundred and five male patients with COVID-19, hospitalized in Imam Hassan Hospital (Bojnurd, Iran), were invited to participate in this cross-sectional study. The required sample size was determined using G Power software (version 3.1.9.4) for the difference between two dependent means. With an effect size of 0.387 based on the study of Karagoz et al. (6), an alpha of 0.05, a statistical power of 0.80, and a probability of 0.7 for dropping out over the 6-month follow-up, the minimum sample size was estimated to be 100. A convenience sampling method was used. The inclusion criteria were self-reported sexual activity before hospitalization, and the exclusion criteria were a history of diagnosed sexual disorders, depression, or anxiety.
The IIEF questionnaire (12) was used at the first visit as well as at the end of months one, three, and six to assess sexual function in each patient. The IIEF is a self-report questionnaire consisting of 15 items and five subscales (erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction). All items were responded to on a Likert scale, with higher scores indicating better sexual functioning. The validity and reliability of its Persian version have been previously evaluated (13).
Descriptive statistical analysis, including mean (SD) for continuous variables and count (percentage) for categorical variables, was used to describe the patient characteristics. Dependent variables included erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. Demographic and clinical variables included age, length of marriage, education level, location, COVID-19 vaccination status, vaccine type, history of comorbidity, and hospitalization duration.
The changes in the dependent variables, as indicators of changes in sexual function over time, were investigated through repeated measures ANOVA with adjustment for age, hospitalization time, and vaccination status. Mauchly's test was performed to check the sphericity assumption. If this assumption was not met, the Geisser-Greenhouse correction was used. Partial eta-squared was applied to estimate effect sizes along with P-values.
The generalized linear model was used to investigate the relationship between sexual disorder indicators and age, length of marriage, education level, location, vaccine status, type of vaccine, and comorbidity through a stepwise approach. All calculations were performed using SPSS version 18.0 (IBM Corp., USA).
4. Results
Out of 105 patients that participated in the study, 9 were lost to follow-up, resulting in an overall follow-up completion rate of 91.4%. No homosexual relations were reported by the patients. The majority of patients had received at least one dose of a COVID-19 vaccine (89.5%). In addition, 23.8% of the patients had a history of comorbidity. Table 1 lists the baseline characteristics.
| Variables | Mean ± (SD) or No. (%) |
|---|---|
| Age (y) | 52.0 ± 14.3 |
| Length of marriage (y) | 29.1 ± 16.9 |
| Education level | |
| Illiterate | 25 (23.8) |
| Primary/high school | 26 (24.8) |
| Undergraduate | 36 (34.3) |
| Postgraduate (Master’s or PhD) | 18 (17.1) |
| Place of residence | |
| City | 78 (74.3) |
| Village | 27 (25.7) |
| COVID-19 vaccination status | |
| Not-vaccinated | 11 (10.5) |
| One dose | 2 (1.9) |
| Two doses | 52 (49.5) |
| Three doses | 40 (38.1) |
| Type of vaccine | |
| Not-vaccinated | 11(10.5) |
| Sinopharm BIBP (China) | 46 (43.8) |
| AstraZeneca Covishield (India) | 9 (8.6) |
| Shifa Pharmed COV Iran Barekat (Iran) | 4 (3.8) |
| Other | 35 (33.3) |
| History of comorbidity a | |
| No | 80 (76.2) |
| Yes | 25 (23.8) |
a The comorbidities included background diseases such as diabetes, cardiovascular diseases and mental illness.
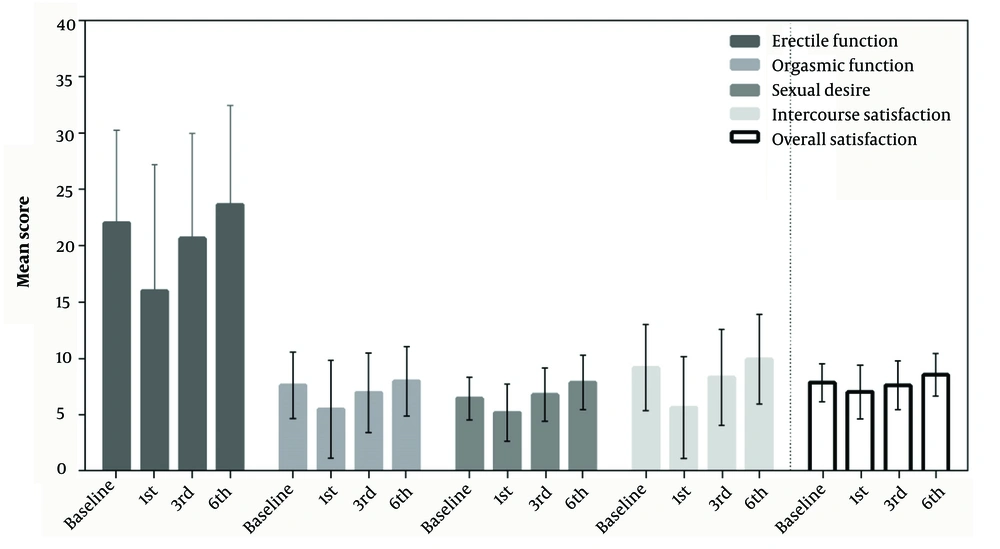
Figure 1 shows the mean (SD) score of the dependent variables at baseline (month 0) as well as at 1, 3, and 6 months after hospitalization. Changes in the scores of orgasmic function (F = 3.034; P = 0.046), sexual desire (F = 6.380; P = 0.001), intercourse satisfaction (F = 3.842; P = 0.015), and overall satisfaction (F = 3.395; P = 0.024) during the follow-up period were statistically significant (P < 0.05). However, erectile function did not show a significant difference during the study (F = 1.075; P = 0.348). Additionally, when comparing the data of each variable at month 6 after hospitalization with that at baseline, orgasmic function (Eta = 0.096; P = 0.003), sexual desire (Eta = 0.163; P < 0.001), and overall satisfaction (Eta = 0.105; P = 0.002) increased significantly 6 months after hospitalization compared to baseline.
The relationship between the demographic data and dependent variables at baseline and the end of the study (month 6) is shown in Appendices 1 - 5 in Supplementary File. From the details in Appendix 1 in Supplementary File, increasing age is associated with lower erectile function at baseline (B = -0.411; P < 0.001) and at month 6 (B = -0.388; P < 0.001). Furthermore, illiterate patients had lower erectile function compared to those with postgraduate degrees (master’s or PhD) at the end of the study (B = -4.049; P = 0.036).
Appendix 2 in Supplementary File shows that orgasmic function decreased with increasing age at baseline (B = -0.033; P = 0.003) and at month 6 (B = -0.131; P < 0.001). Moreover, older people experienced a greater decline in orgasmic function during the follow-up period (B = -0.056; P = 0.001). Additionally, the length of marriage was related to the decrease in orgasmic function only at baseline (B = -0.089; P < 0.001).
From Appendix 3 in Supplementary File, sexual desire decreased with increasing age at baseline (B = -0.094; P = 0.003) and at month 6 (B = -0.108; P < 0.001). Additionally, patients with an illiterate education level had lower sexual desire compared to those with a postgraduate level at baseline (B = -0.984; P = 0.029) and at month 6 (B = -1.852; P = 0.023).
A significant negative relationship was also observed between intercourse satisfaction and age at baseline (B = -0.179; P < 0.001) and at month 6 (B = -0.177; P < 0.001). Illiterate patients had a lower level of intercourse satisfaction than patients with postgraduate education at baseline (B = -1.906; P = 0.040) and at 6 months (B = -2.717; P = 0.011) (see Appendix 4 in Supplementary File).
Furthermore, from Appendix 5 in Supplementary File, an increase in age was associated with a decrease in overall satisfaction at both time points (B = -0.009; P = 0.016 at baseline and B = -0.073; P < 0.001 at month 6).
The length of marriage was related to a decrease in overall satisfaction at baseline (B = -0.050; p < 0.001), but not at month 6. Patients with a postgraduate degree showed higher overall satisfaction levels compared to other groups, both at baseline and at six months. Additionally, those living in the city had higher overall satisfaction compared to villagers (B = 1.959; p < 0.001) at baseline.
5. Discussion
Catastrophic events that create chaotic and traumatic environments can have short- and long-term psychological effects on couples (14), potentially affecting their sexual behavior. COVID-19, as a recent disaster, has had negative effects on various aspects of health, including anxiety, depression, and sexual disorders (15), and has played an important role in changing sexual behavior in couples. Sexual behavior is related to the intimate relationship between couples, sexual desire, and emotional intimacy (16), and broadly highlights the degree of interdependence between couples (17). During the COVID-19 pandemic, there has been significant mental and physical stress, which could lead to changes in sexual behavior, such as a decrease in casual sex and sexual enjoyment/pleasure (18). Anxiety, stress, and fear of the disease were likely the main causes of changes in sexual life (19).
Apart from the impact of the COVID-19 pandemic on everyday sexual lives, infection with COVID-19 could have a more pronounced effect on sexual relations. In this cross-sectional study, we examined the effect of COVID-19 infection on sexual function in men. Five indicators (orgasm function, erection function, sexual desire, sexual satisfaction, and overall satisfaction) were studied at months 0, 1, 3, and 6 post-infection. Findings from the present investigation indicated that the scores in all five variables declined during the first month after diagnosis compared to baseline. After the first month, the variables appeared to improve.
This difference was statistically significant, except in the case of erectile function. Other studies have shown a negative impact of COVID-19 on men's sexual performance. For example, the prevalence of erectile dysfunction in COVID-19 patients was 33%, significantly higher than that of the general population (9%). Anxiety (8) and major depression during the acute phase of infection (11) have been reported as possible risk factors for erectile dysfunction.
The only report we could find on the long-term effects of COVID-19 on sexual behavior was by Harirugsakul et al. The study reported improvement in erectile dysfunction and IIEF scores after three months. It showed that erectile dysfunction in the third month after recovery was associated with age > 40 years (11), similar to our study.
Our findings indicated that after the first month of infection, sexual dysfunction gradually improves. It is well-known that sexual function is closely associated with physical and psychological conditions (20). Previous reports suggest that symptoms such as changes in smell and taste (21) are expected to improve gradually within a few months, while psychological outcomes such as PTSD, anxiety, and depression (22) and cognitive functions (23) may take longer than six months to improve. Therefore, it is arguable that sexual dysfunction in COVID-19 is due to factors other than psychological changes.
It has been suggested that factors such as the infiltration of the virus into organs, dysregulation of peripheral cytokines, autoimmune diseases following infection, and the translocation of gut microbes could contribute to the manifestation of COVID-19 in other organs (24). In terms of the effects of COVID-19 on the reproductive system, very limited data are available. COVID-19 mRNA has been detected in seminal fluid. Additionally, male gonadal hypersensitivity to COVID-19 has been observed, likely due to higher expression of angiotensin-converting enzyme 2 (ACE-2) receptors (3). The possibility of testicular involvement in COVID-19 has also been suggested by reports of testicular pain (25). To the best of our knowledge, no long-term studies are available on COVID-19 and the reproductive system. For instance, we could not find data about inflammation, the presence of the virus, etc., in the testis after six months of recovery from COVID-19. Therefore, we believe that further studies are required to investigate the mechanisms underlying the improvement in sexual dysfunction after the first month of infection.
In this study, we did not find a direct relationship between demographic data and COVID-19. However, education level and age appear to influence sexual function, similar to findings in previous literature. For instance, the general health of elderly patients has been associated with common challenges in sexual function (26). In another report, a lower level of education has been linked to decreased sexual function (27). It is also important to note that confounding variables (e.g., medication regimen and disease severity) could potentially affect the findings of our study and should be considered in future investigations.
5.1. Conclusions
In general, this short-term and long-term study found that patients with COVID-19 infection may experience sexual dysfunction from the time of infection to a few months after recovery.