1. Background
Methamphetamine (MA) is a psychotropic substance known for its widespread abuse, which is attributed to the feelings of euphoria, improved mood, and increased self-confidence it produces. The drug's accessibility through simple synthesis methods, cost-effectiveness, and rapid onset of action have contributed to its rise as the second most commonly used illicit substance class worldwide (1-3). Several pathways have been implicated in the neurotoxic effects of MA initiated by excessive dopamine release, including damage to monoaminergic terminals (4), apoptosis (5), neuroinflammation (6), and oxidative stress (7). One of the most important mechanisms underlying the neurotoxicity of MA is the induction of oxidative stress, which leads to significant generation of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radicals (OH-), and superoxide anion (O2-), by facilitating dopamine (DA) oxidation, ultimately causing harmful effects on RNA, DNA, proteins, and lipids (8, 9). On the other hand, the antioxidant system acts as a protective mechanism against free radicals, shielding neuronal cells from oxidative damage (10).
Preclinical studies have shown that both acute and chronic exposure to MA leads to neurodegeneration and oxidative stress in the prefrontal cortex, hippocampus, and limbic system. Changes in these areas are associated with emotional and cognitive dysfunction, such as anxiety, depression, learning, and memory deficits (11-14). Approximately 27% of people who use stimulants may experience impairment in various cognitive domains, which may be associated with psychosis and changes in social function (15, 16).
Rutin, known as 3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside, is classified as a biophenolic flavonoid compound that is widely distributed in a variety of plants and fruits, including tea, buckwheat, and apples. In addition, this compound serves as an important nutritional component found in a variety of foods and plant-based beverages (17). Various animal studies have demonstrated Rutin's ability to provide a spectrum of neuroprotective benefits through its role as an antioxidant. These benefits include anticonvulsant properties in the pentylenetetrazol model (18), attenuation of neuroinflammation in the stroke model (19), improved motor behavior in hyperkinetic movement disorder (20), support of dopaminergic neuron survival in Parkinson's disease (21), and memory enhancement (22). In addition, studies in Alzheimer's disease have shown that rutin may improve cognitive function by preventing neuroinflammation and oxidative stress by reducing levels of tau pathology and Aβ oligomers (23-25).
2. Objectives
In the present study, we investigated the potential neuroprotective effects of rutin on MA-induced anxiety-related behavior and cognitive dysfunction.
3. Methods
3.1. Animals
This experimental study was conducted on adult male Wistar rats weighing approximately 200 to 250 grams. The rats were housed in groups of four in plastic cages in the animal facility of Mazandaran Institute of Medical Sciences, with free access to food and water, at a temperature of 23 ± 2°C and humidity of 50 ± 10%, under a 12:12-hour dark-light cycle. The Research Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran, approved all experiments (IR.MAZUMS.REC.1398.1382).
3.2. Drugs
Methamphetamine hydrochloride (with 99.2% purity) was synthesized at the Medical Chemistry Laboratory of the University of Medical Sciences, Tehran, Iran. It was freshly dissolved in 0.9% saline prior to each subcutaneous injection. Rutin (CAS No. 153-18-4) was purchased from Sigma and dissolved in sterile, pyrogen-free 0.9% saline prior to intracerebroventricular (ICV) injection in a volume of 5 μL. Ketamine (Rotexmedica, GmbH, Germany) and xylazine (Loughrea Co, Galway, Ireland) were administered intraperitoneally (IP).
3.3. Surgery Procedures
After one week of acclimatization in the animal facility, the rats were anesthetized with a ketamine-xylazine mixture (80 and 20 mg/kg, IP, respectively) and then fixed in a stereotaxic frame (Stoelting, USA) for ICV injection into the right lateral ventricle of the brain. The cannula (30 gauge) was placed according to the Watson and Paxinos Atlas coordinates (AP: -0.5, ML: 1.6, DV: -4.2) (26) and secured to the skull with dental cement. All ICV injections were performed over a 5-minute period using a 30-gauge stainless steel injector connected to a Hamilton syringe via polyethylene tubing (PE-20).
3.4. Experimental Design
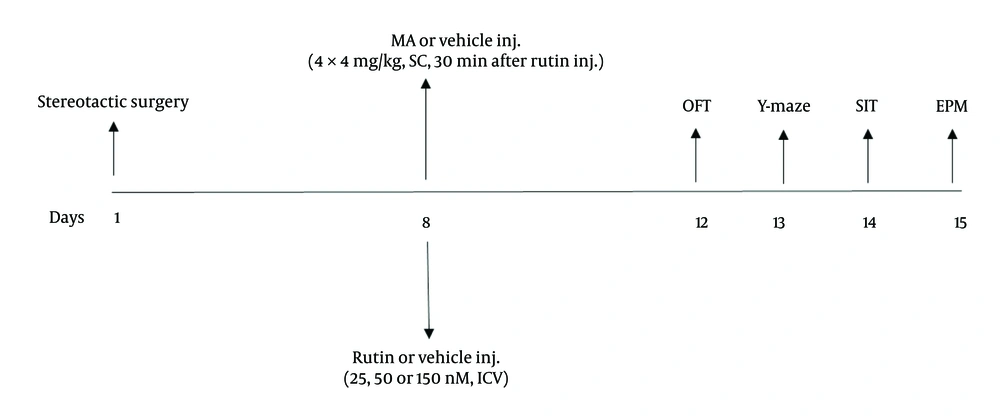
The rats were randomly divided into six groups after a 7-day postoperative recovery period: (1) Control group, which received a single ICV injection of normal saline (5 µL/right ventricle) followed by subcutaneous administration of normal saline 30 minutes later; (2) MA group, which received a single ICV injection of normal saline (5 µL/right ventricle) followed by subcutaneous administration of MA (4 mg/kg, with a total of 4 injections at 2-hour intervals) 30 minutes later; (3, 4, 5) treatment groups (MA+rutin), which received a single ICV injection of three different doses of rutin (25, 50, or 150 nM, 5 µL/right ventricle, respectively) followed by subcutaneous administration of MA (4 mg/kg, with a total of 4 injections at 2-hour intervals) 30 minutes later; (6) rutin group, which received a single ICV injection of rutin (150 nM, 5 µL/right ventricle) followed by subcutaneous administration of normal saline 30 minutes later. Behavioral assessments, including open field, Y-maze, social interaction, and elevated plus maze, were conducted three days later (Figure 1). The behavioral tests were conducted between 9:00 AM and 3:00 PM. Furthermore, the behavioral equipment was cleaned with a solution of 70% ethanol after each individual assessment.
3.5. Body Temperature
The body temperature of the animals was measured with an oiled rectal probe connected to a digital thermometer (Braun GmbH, Germany). This measurement was performed 1 hour after the last MA administration. The rectal probe was carefully inserted into the rectum to a depth of 2 cm and held there for 40 seconds to determine core body temperature (27).
3.6. Behavioral Tests
3.6.1. Open Field Test
The locomotor frequency of the rats was measured during the open field test (OFT). The test took place in a square Plexiglas box measuring 100 × 100 × 40 cm, which was divided into 4 × 4 smaller squares of equal size by thin black lines. The test lasted 5 minutes. At the beginning, the rat was placed in the center of the box, and all its movements were recorded by a camera mounted above the apparatus. At the end of the test, all crossed lines were measured by a blinded experimenter (28).
3.6.2. Y-maze Spontaneous Alternation Test
The Y-maze test was used to assess spatial working memory. This test consists of three arms arranged in a "Y" shape, with each arm at a 120° angle to the others. During the test, the rats were allowed to freely explore the arms for a period of 8 minutes. The experiment recorded the number of entries, defined as the entry of all four paws into each arm, as well as alternation, which measures multiple entries into the three arms (A, B, or C) on three overlapping sets. To capture the behavior of the rats, a camera was placed above the apparatus, and the collected data was later analyzed by an experimenter blinded to the conditions (11).
3.6.3. Social Interaction Test
The social interaction test (SIT) was performed to assess the sociability and social memory of the animals. For this behavioral test, a rectangular Plexiglas box divided into three chambers (40 × 40 × 40 cm) with sliding doors was used. Each of the two side chambers contained a small cage. The experimental procedure consisted of three consecutive 10 min sessions:
3.6.3.1. Acclimatization Session
The rat was placed in the central chamber after the sliding doors were opened, allowing the rat to freely explore all three chambers. Two side chambers were equipped with small empty cages.
3.6.3.2. Sociability Session
An age- and sex-matched rat (referred to as stranger 1) was placed in a small cage in one of the side chambers, while the opposite chamber was occupied by an empty cage. The partitions between the chambers were then removed, giving the rat unrestricted access to all chambers. The time of direct contact between the test rat and stranger 1 was documented. The stranger was randomly placed in either the left or right chamber of the social test box to avoid chamber preference.
3.6.3.3. Social Memory Session
During the social memory session, an experimental rat was placed in the central chamber alongside another rat of similar age and sex (referred to as stranger 2), which was placed in a small, previously unoccupied cage. Meanwhile, "stranger 1" was housed in a small cage located in a distinct chamber. The sliding doors were activated, allowing the rat in the central chamber access to the left and right chambers. The duration of direct interaction between the experimental rat and the two stranger rats was recorded by a digital camera positioned above the setup to capture the social interactions of the rats, and the data was subsequently analyzed by a blinded experimenter (29).
3.7. Elevated Plus Maze Test
The elevated plus maze (EPM) test was used to evaluate anxiety-related behavior in rats. The apparatus consisted of two closed arms (50 × 10 × 50 cm) and two open arms (50 × 10 cm), with a central platform (10 × 10 cm) raised 50 cm above the ground. Each rat was carefully positioned in the center of the EPM, facing one of the open arms. Throughout the 8-minute trial, the rats' activities were recorded by a digital camera placed above the maze. The number of entries and time spent in both open and closed arms were meticulously documented (30).
3.8. Statistical Analysis
Results are presented as the mean ± SEM and analyzed using the 27th version of SPSS software. Paired t-test and Kruskal-Wallis test were used to determine significance between groups, and the Dunn-Bonferroni post hoc test was also used for pairwise comparisons. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Effects of Different Concentrations of Rutin on Body Temperature in MA-Treated Rats
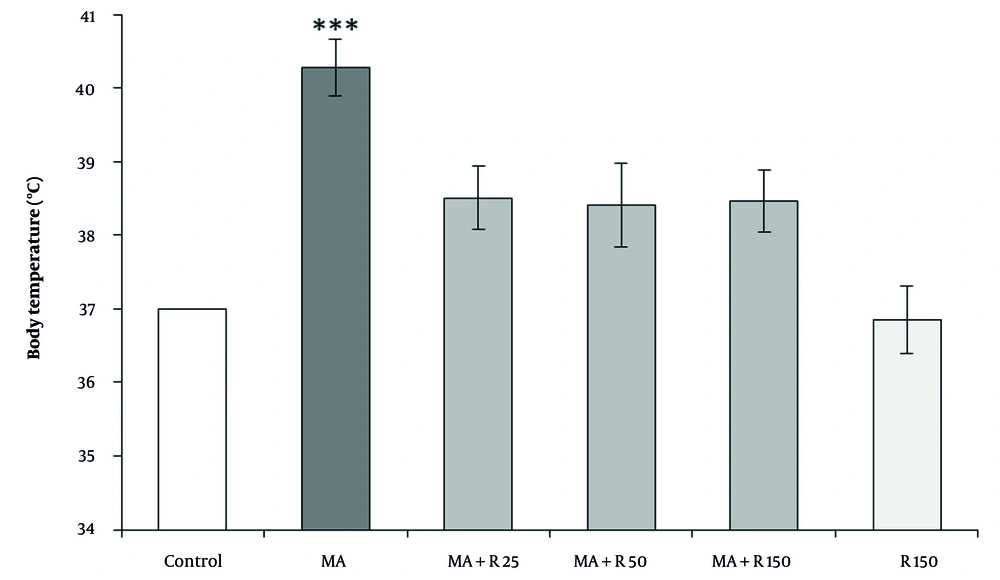
As shown in Figure 2, a significant difference in body temperature was observed between the experimental groups using the Kruskal-Wallis test. Dunn-Bonferroni post hoc test results showed that MA administration produced hyperthermia compared to the control group at ambient temperature (P < 0.001). Pretreatment with three different concentrations of rutin (25, 50, and 150 nM) reduced body temperature compared to the MA group, but this decrease was not statistically significant (P > 0.05). No significant difference in body temperature was observed after injection of rutin (150 nM) alone compared to the control group (P > 0.05). It should be noted that no statistically significant difference was observed between the groups that received MA along with different doses of rutin (P > 0.05).
The effects of MA (4 × 4 mg/kg, at 2 h intervals) and different concentrations of rutin (25, 50, and 150 nM) on body temperature. Statistical analysis was performed using Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test to determine differences between groups. Methamphetamine (MA) administration produced hyperthermia compared to the control group (P < 0.001). Whereas, pretreatment with three different doses of rutin did not cause a statistically significant decrease in body temperature compared to the MA group (P > 0.05). Data are presented as mean ± SEM (n = 8 - 10). *** P < 0.001 vs. control group.
4.2. Effects of Different Concentrations of Rutin on Locomotor Activity in MA-Treated Rats
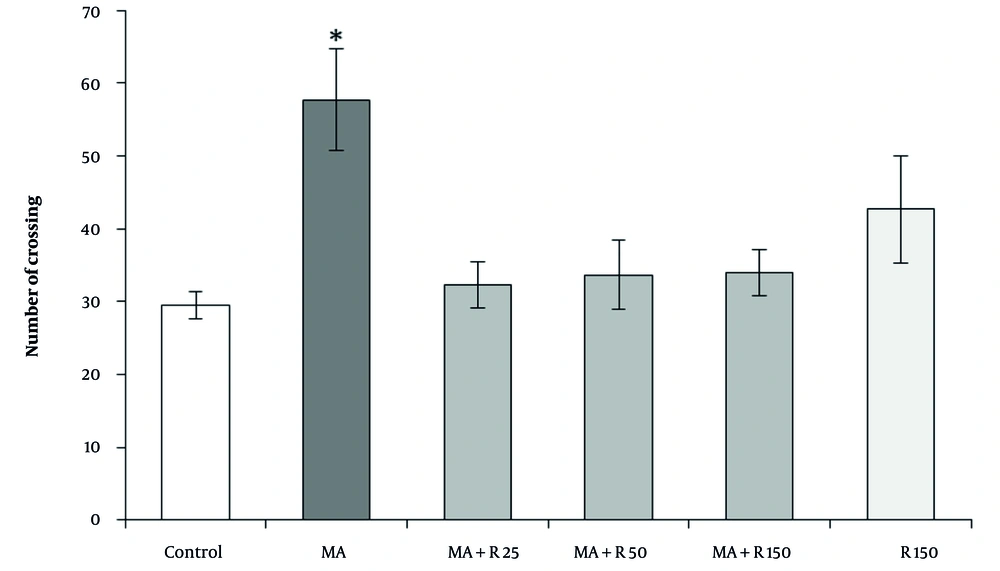
The results of the Kruskal-Wallis analysis revealed a significant difference between the groups in the number of crossings in the OFT. Dunn-Bonferroni post hoc results indicated that the MA-treated group crossed the line more frequently than the control group (P < 0.05, Figure 3). Treatment with three different doses of rutin (25, 50, and 150 nM) decreased the number of crossings compared to the MA group, but this decrease was not statistically significant (P > 0.05). There was no statistical difference in the number of crossings between the rutin (150 nM) alone group compared to the control group (P > 0.05). In addition, no statistically significant difference was observed between the groups that received MA along with different doses of rutin (P > 0.05).
The effects of Methamphetamine (MA) (4 × 4 mg/kg, at 2 h intervals) and different concentrations of rutin (25, 50, and 150 nM) on locomotor activity in the open field test. Statistical analysis was performed using Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test to determine differences between groups. Methamphetamine increased the number of crossings compared to the control group (P < 0.05). Rutin injection at three different doses did not cause a statistically significant reduction in number of crossings compared to the MA group (P > 0.05). Data are presented as mean ± SEM (n = 8 - 10). * P < 0.05 vs. control group.
4.3. Effects of Different Concentrations of Rutin on Spontaneous Alternation Behavior in MA-Treated Rats
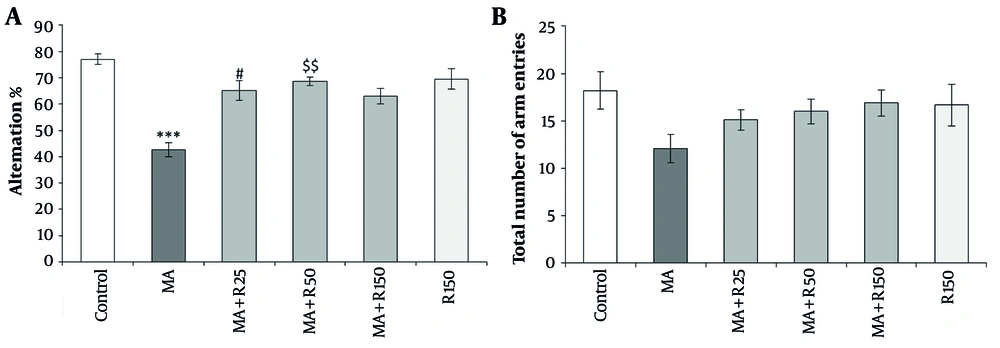
There was a significant difference between the groups on spatial working memory in the Y-maze test. Dunn-Bonferroni post hoc results revealed a significant decrease in spontaneous alternation in the MA-treated group compared to the control group (P < 0.001, Figure 4A). On the other hand, rutin injection at two different concentrations (25 and 50 nM) reversed the spatial working memory deficit induced by MA (P < 0.05 and P < 0.01, respectively). In addition, drug-induced locomotor alternations, which can affect alternation rates, were assessed by counting the total number of entries in the Y-maze arms during the 8-minute trial. However, all animals showed equal locomotor activity (P > 0.05, Figure 4B). No statistically significant difference was observed between the groups that received MA along with different doses of rutin (P > 0.05).
The effects of Methamphetamine (MA) (4 × 4 mg/kg, at 2 h intervals) and different concentrations of rutin (25, 50, and 150 nM) on working memory in the Y-maze test. (a) Alternation % and (b) total number of arm entries. Statistical analysis was performed using Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test to determine differences between groups. MA reduced spontaneous alternation compared to the control group (P < 0.001). Whereas, pretreatment with rutin (25 and 50 nM) increased spontaneous alternation compared to the MA group (P < 0.05 and P < 0.01, respectively). The total number of arm entries was not significant among of experimental groups (P > 0.05). Data are presented as mean ± SEM (n = 8 - 10). *** P < 0.001 vs. control group, # P < 0.05 and $$ P < 0.01 vs. MA group.
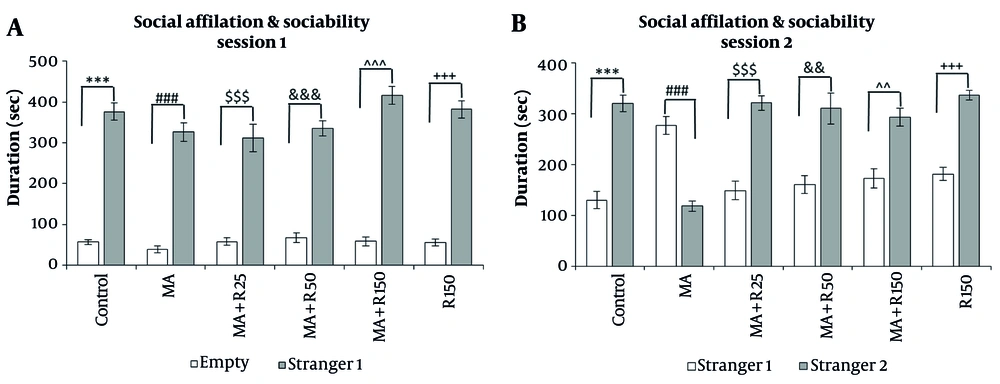
4.4. Effects of Different Concentrations of Rutin on Social Behavior in MA-Treated Rats
During session 1, we measured the sociability of the experimental groups by observing the amount of time spent with either a stranger or an empty cage. We found that regardless of drug administration, all experimental groups spent more time with the stranger than with the empty cage (P < 0.001, Figure 5A), indicating that sociability was not affected by the treatments. During session 2, we focused on social memory and novelty. We found that the MA-treated group spent less time exploring Stranger 2 compared to stranger 1 (P < 0.001, Figure 5B), indicating that they had impaired social memory. However, when MA-treated rats were given different doses of rutin (25, 50, and 150 nM), they spent significantly more time exploring Stranger 2 than Stranger 1 (P < 0.001, P < 0.01, P < 0.01, respectively). The 150 nM dose of rutin had the same effect as the control groups (P < 0.001).
The effects of MA (4 × 4 mg/kg, at 2 h intervals) and different concentrations of rutin (25, 50, and 150 nM) on A, sociability; and B, social memory in the social interaction test. Statistical analysis was performed using paired t-test. During session 1, all experimental groups spent more time with the stranger 1 than the empty cage (P < 0.001). During session 2, MA-treated group spent less time exploring stranger 2 compared to stranger 1 (P < 0.001). Whereas, pretreatment with three different doses of rutin (25, 50, and 150 nM) increased the exploration time of stranger 2 compared to stranger 1 (***, ###, $$$, ^^^, +++, &&& P < 0.001, ^^ and && P < 0.01, * P < 0.01, respectively). Data are presented as mean ± SEM (n = 8 - 10).
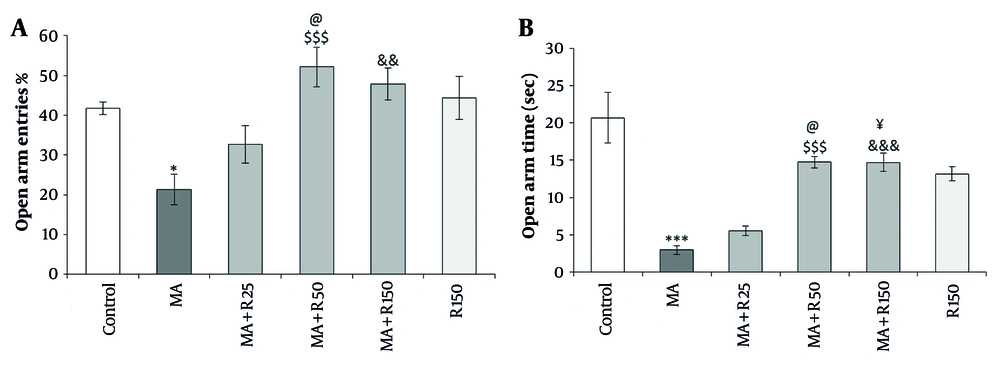
4.5. Effects of Different Concentrations of Rutin on Anxiety-Related Behavior in MA-Treated Rats
According to Figure 6A and B, Kruskal-Wallis analysis showed that there was a significant difference in the percentage of open arm entries and open arm time between the groups. Further analysis using the Dunn-Bonferroni post hoc test indicated that the MA group spent less time in open arms (P < 0.001) and made fewer entries into open arms (P < 0.05) compared to the control group. Administration of rutin at doses of 50 nM and 150 nM resulted in a decrease in the anxiogenic response induced by MA, resulting in more time spent (P < 0.001) and entries into open arms (P < 0.001 and P < 0.01, respectively). The response of the rutin group at a dose of 150 nM was not significant compared to the control group for both factors measured by the EPM (P > 0.05). Pairwise comparison also showed that animals that received MA along with the dose of 50 nM rutin showed a significant difference compared to animals that received MA along with the dose of 25 nM rutin in the open arm entries (P < 0.05). In addition, in the open arms time parameter, animals that received MA along with the doses of 50 or 150 nM rutin showed a significant difference compared to animals that received MA along with the dose of 25 nM rutin (P < 0.05).
The effects of MA (4 × 4 mg/kg, at 2 h intervals) and different concentrations of rutin (25, 50, and 150 nM) on A, open arm entries; and B, open arm time in the The elevated plus maze (EPM) test. Statistical analysis was performed using Kruskal-Wallis test followed by Dunn-Bonferroni post hoc test to determine differences between groups. MA decreased open arm entries (P < 0.05) and open arm time (P < 0.001) compared to the control group. Whereas, pretreatment with rutin (50 and 150 nM) increased open arm entries (P < 0.001 and P < 0.01, respectively) and open arm time (P < 0.001) compared to the MA group. Data are presented as mean ± SEM (n = 8 - 10). * P < 0.05 and *** P < 0.001 vs. control group, $$$ P < 0.001, && P < 0.01, and &&& P < 0.001 vs. MA group, @ P < 0.05 and ¥ P < 0.05 vs. MA+R25 group.
5. Discussion
The current investigation showed that pretreatment with rutin attenuated MA-induced hyperthermia and hyperlocomotion, but this reduction was not statistically significant. Furthermore, rutin effectively improved spatial working memory and social memory deficits induced by MA. In addition, rutin led to a reduction in MA-induced anxiety-related behaviors. Our results showed that the dose of 50 nM rutin performed better than doses of 25 and 100 nM in reducing behavioral disturbances caused by MA.
Numerous pathways have been documented to contribute to MA-induced neurotoxicity, including hyperthermia (31), oxidative stress (8), apoptosis, dopaminergic and serotonergic terminal impairment, excitotoxicity, and neuroinflammation (32). One of the primary mechanisms underlying the neurotoxic effects of MA is oxidative stress. Methamphetamine's ability to penetrate the plasma membrane of dopaminergic neuron terminals and interact with the dopamine transporter facilitates increased release of dopamine into the synaptic cleft (33). The auto-oxidation of excess dopamine leads to the production of reactive nitrogen species (RNS) and ROS on a significant scale, resulting in dysfunction of both the mitochondria and the endoplasmic reticulum. The escalation of oxidative stress markers leads to neurodegeneration of dopaminergic terminals and apoptosis (32). Numerous studies have shown that an increase in ROS and an imbalance between antioxidant capacity and oxidative stress in the central nervous system contribute to neurodegeneration, resulting in the development of conditions that promote abnormal behavior and memory loss (34-36).
The studies provided evidence that rutin, a type of flavonoid, has a neuroprotective effect against several brain disorders, including Parkinson's disease, Alzheimer's disease, stroke, and seizures (19, 23, 37, 38). Rutin has the potential to therapeutically alleviate brain diseases by exerting anti-inflammatory (39), antioxidant (40), and anti-apoptotic effects (41). Currently, there are few comprehensive studies examining the effects of rutin on addiction, particularly focusing on alcoholism. Subedi et al. found that rutin dose-dependently ameliorated cognitive impairment induced by ethanol in rats. This improvement was achieved through the antioxidant effects of rutin on the hippocampus and cortex (42). It has also been reported that oral administration of rutin reduces ethanol reinforcement in mice (43), but in the case of MA dependence, this study is the first to investigate the neuroprotective effects of rutin on MA-induced neurobehavioral and memory impairment.
Our study showed that rutin partially reduced the body temperature induced by the neurotoxic dose of MA. Hyperthermia enhances the neurotoxic effects of MA by promoting the generation of ROS (44); therefore, it is believed that the antioxidant properties of rutin have an effect on reducing the neurotoxic effects of MA and mitigating cognitive decline in rats exposed to MA by reducing body temperature. Rutin has also been demonstrated to partially reduce the intensity of MA-induced behavioral sensitization, such as hyperlocomotion. In line with our findings, several studies have shown that natural compounds such as hispidulin and sauchinone, derived from Clerodendrum inerme and the root of Saururus chinensis, respectively, can reduce hyperlocomotion induced by MA (45, 46). In contrast, another study showed that pretreatment with another flavonoid, baicalein, did not affect hyperlocomotion induced by acute MA injection in mice (47).
This study does not examine the specific biochemical and molecular pathways by which rutin affects the neurobehavioral changes caused by MA. Therefore, further studies are needed to investigate the ability of this flavonoid to mitigate the neurochemical and molecular consequences associated with MA.
In this study, we also found that an acute binge dose of MA injection impaired social memory but not sociability in the social interaction test and elicited anxiety-related behavior in the EPM. Anxiety and social functioning are two traits that are affected by MA. In addition, there is evidence that those with higher levels of anxiety show greater deficits in social interaction, suggesting a possible correlation between the two behaviors (48). Furthermore, it appears that long-term use of MA is necessary to impair sociability, and that acute use does not cause harm, so that antisocial behaviors such as violence and aggression may be seen in people who chronically use MA (49, 50).
According to our findings, rutin at three different doses had no effect on sociability in the MA-treated groups. However, it improved the social memory and spatial working memory deficits caused by MA in the social interaction and Y-maze tests. This suggests that rutin has a memory-enhancing effect on cognitive function. Consistent with our research, Ishola et al. demonstrated that pretreatment with various doses of rutin reduced impairments in working memory and spatial learning in mice exposed to scopolamine. This was achieved by enhancing antioxidant defenses and cholinergic systems in the hippocampus and prefrontal cortex (51). A large body of literature has shown the beneficial effects of rutin on many types of memory, including episodic memory, passive avoidance memory, and spatial memory, in animal models of cognitive deficits (52-55). It is also suggested that the beneficial effects of rutin on memory function may be due to its ability to reduce anxiety. As mentioned above, rutin was found to reduce anxiety levels in rats treated with MA. Anxiety has been reported to affect both short-term and long-term memory, suggesting a link between anxiety and cognitive function (56).
Although several studies have shown that MA causes neurobehavioral disorders, the effects of rutin on MA-induced neurotoxicity have not been investigated. In this preliminary study, we only investigated the neuroprotective effects of rutin on MA-induced anxiety-related behaviors and cognitive dysfunction. Therefore, there is a pressing necessity to evaluate the therapeutic potential of rutin in reducing MA-induced neurotoxicity at the molecular and histological levels in different brain regions.
5.1. Conclusions
We found that pretreatment with rutin partially alleviated the symptoms of hyperthermia and hyperlocomotion induced by MA. Furthermore, it effectively reduced the social memory impairment and working memory deficit in rats exposed to MA by exerting anxiolytic properties. These findings suggest that the inclusion of rutin in the diet can provide protection against neurobehavioral and cognitive dysfunction induced by MA. Furthermore, these results provide valuable insight into the potential use of natural products as a medicinal approach for the development of therapeutic methods to combat MA neurotoxicity.