1. Background
Major depressive disorder is one of the most common causes of disability in people of the world, so it has imposed a heavy burden on society in terms of medicine and the economy (1). So far, the molecular causes of major depressive disorder (MDD) remain largely unknown, and our understanding of the relationship between various molecular pathways and MDD is incomplete. When evaluating biomarkers related to the disorder, targeting multiple molecular pathways may prove beneficial (2). The MDD diagnosis and treatment process is complicated due to the disease’s inherent complexity, limited diagnostic criteria, and the absence of validated biomarkers, which further hinder the accurate identification and effective treatment of patients (3).
The bioinformatics objective of these studies is to identify and introduce appropriate non-invasive biomarkers for patients with MDD (4). Overall, the utilization of RNA and clinical data is crucial for identifying and understanding the pathogenesis of MDD. Despite being the most dynamic component in disease evaluation and offering a wealth of information, RNA is underutilized in supporting clinical diagnosis (5).
There is a tripartite relationship among depression, decreased gene expression of the FKBP5 (FK506 Binding Protein 5), increased gene expression of glucocorticoid receptor (GR), and hypothalamic-pituitary-adrenal (HPA) axis function. Changes in the expression of each of these genes affect depression (6). The serotonin transporter, encoded by the solute carrier family 6 member 4 (SLC6A4) gene, regulates the extracellular levels of serotonin. The SLC6A4 gene encodes the serotonin transporter and regulates the serotonin extracellular level. A reduction in the SLC6A4 gene expression leads to decreased serotonin levels in the synaptic cleft, contributing to the pathogenesis of depression and forming the basis of the monoaminergic theory (7).
A previous study (Mohammadi, 2022) identifies the DNA methylation status of FKBP5 and SLC6A4 genes associated with MDD following treatment with selective serotonin-reuptake inhibitors (SSRIs). Based on the proven effect of antidepressants on DNA methylation and gene expression, these medications can improve the treatment process and reduce depression scores post-treatment (8). Research efforts have focused on psychiatric disorders, with a significant emphasis on the roles of the FKBP5 and SLC6A4 genes in the pathobiology of MDD. One of the important and valuable biomarkers in identifying major depressive disorder is the FKBP5 and SLC6A4 genes in depressed patients. It has been observed that the expression of these genes may be potentially diminished in patients with MDD (9). When evaluating responses to treatment through pharmacological and psychotherapeutic methods, conflicting results have emerged. It is believed that these treatment approaches do not lead to changes in gene expression (10, 11). Conversely, several studies hold the view that both pharmacotherapy and psychotherapy can indeed alter gene expression in patients with mental disorders (8, 12).
To our knowledge, there are no published studies that report the sensitivity, specificity, and post-treatment changes of the FKBP5 and SLC6A4 biomarkers in two concurrent treatments within a randomized clinical trial involving patients with MDD.
2. Objectives
This study aimed to investigate the sensitivity and specificity of FKBP5 and SLC6A4 genes as biomarkers between MDD patients and healthy controls (HCs) and the response to treatment with cognitive behavioral therapy and fluoxetine therapy in a randomized clinical trial.
3. Methods
3.1. Study Design and Participants
The present study was conducted as a parallel randomized clinical trial. This study was approved by the Research Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (approval code: IR.MAZUMS.REC.1398.759) approval date: 2019.08.07. The study protocol was registered in the Iranian Registry of Clinical Trials (IRCT) (IRCT20190710044171N1). Additionally, the study was conducted in accordance with the principles of the Helsinki Declaration.
Inclusion criteria included a diagnosis of MDD based on the DSM-V criteria, being in the age group of 18 to 65 years, having a Beck Depression Inventory-II (BDI-II) depression score ≥ 20 (i.e., moderate to severe depression), not taking antidepressants for at least 2 weeks (or 4 weeks if previously taking fluoxetine), lack of previous psychotherapy (2 weeks), and conscious willingness to participate in the research. Exclusion criteria included acute psychiatric disorders during the research period, having serious and limiting medical diseases during the research, not attending more than two consecutive sessions in cognitive behavioral therapy (CBT), not taking medicine regularly for two weeks, having renal or hepatic impairments, heart disease, glaucoma, or pregnancy.
3.2. Sample Size
To determine the sample size, the quantitative variable of serotonin transporter gene expression (SLC6A4) was used, which was the result of fluoxetine administration in patients with MDD in Tsao et al.’s study (13). In this study, the mean and standard deviation of SLC6A4 gene expression levels were 1.25 ± 0.5 and 0.63 ± 0.3 before and after fluoxetine administration, respectively. Based on these values, the sample size of 7 participants in each group, with a 95% probability and a 99% confidence level, was determined to be adequate for rejecting the null hypothesis of equal effects before and after the study.
Considering the possibility of dropping out and increasing the power of the study, the sample size was increased to 20 participants in each group. Therefore, a total of 40 patients with major depression were included in this study.
All participants in the present study were patients from private clinics and Zare Hospital in Sari from January 2022 to March 2022. Major depressive disorder patients (n = 40) were identified based on the diagnostic and statistical manual of mental disorders-fifth edition (DSM-V) criteria and a clinical interview conducted by a specialist (14).
The control group was selected from students and employees through psychiatric interview, so that if they did not have a history of psychiatric disorder, they would be included in the control group (n = 44). They were screened by an expert using the Patient Health Questionnaire-9 (PHQ-9) to rule out mental disorders and other confounding factors (15).
To control and eliminate bias and ensure the concealment of allocation and compliance with the randomization rule in the randomized clinical trial method, patients were included in the study based on the inclusion and exclusion criteria. They were divided into two equal groups of CBT and fluoxetine therapy by an expert who was not aware of the research objectives. Patients were followed up weekly in terms of confounding factors such as other drugs, psychotherapy, and nutrition. Statistical analysis was performed by an independent statistician.
Depression scores of patients and HCs were evaluated using BDI-II (16). Initially, samples from MDD patients and HCs were collected and analyzed. Patients in the CBT group (n = 20), group sessions were held for 90 minutes for 12 consecutive weeks under the supervision of a psychiatrist. For Patients in the fluoxetine therapy group (n = 20) were given 20 mg of fluoxetine daily for three months and followed up weekly for drug use. After the treatment period, sampling and analysis were repeated, and the BDI-II score was evaluated post-intervention. The BDI-II: This scale is the revised version of the BDI, which was developed to measure an individual’s depression level through 21 items. The four choices of each question are scored in a four-level spectrum from 0 to 3. Therefore, the total score of the questionnaire ranges from 0 to 63. The internal consistency of the inventory is reported to be 0.91 (17) The psychometric properties of the inventory in Iran are reported as Cronbach’s alpha coefficient of 0.91, a correlation coefficient of 0.89 between the two halves, and a one-week retest coefficient of 0.94. The Dimensional Anhedonia Rating Scale (DARS) also demonstrated good convergent and divergent validity with Snaith-Hamilton Pleasure Scale (SHAPS) (18, 19).
To ensure the concealment of allocation and compliance with the rule of randomization and to reduce bias in the random clinical trial method and sample size, patients were equally divided into a CBT group and a fluoxetine group by an expert who was unaware of the research objectives. An independent statistician performed a statistical analysis.
3.3. RNA Extraction and cDNA Synthesis
We collected 5 mL of peripheral blood from both MDD subjects and HCs, using tubes prepared with ethylenediaminetetraacetic acid (EDTA) anticoagulant. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll and density gradient centrifugation. RNA extraction was carried out as per the Gene AII Kit instructions (Cat: 300-001). cDNA was synthesized following the instructions of the Pars Tous Kit (Cat: A1001161).
Synthesis of cDNA from template mRNA: Kit components and template mRNA were added to the RNase-free tube and brought to volume with diethyl pyrocarbonate (DEPC)-treated water. The oligo primer (dt) connects to the polyA part of the template strand (mRNA) and creates a free 3-OH end. It is amplified in the presence of the reverse transcription enzyme [H-minus Moloney murine leukemia virus (MMLV)] and deoxynucleoside triphosphate (dNTP), and single-stranded cDNA is produced. The random hexamer (6 nucleotides) is randomly attached to parts of mRNA and synthesizes the cDNA molecule in the presence of the enzyme (H-minus MMLV) and dNTP. The reactions were performed according to the temperature program (47 and 85 degrees) using of thermal cycler. According to the kit brochure, primer oligo dt, H-minus MMLV enzyme, template strand (mRNA), and random 6-nucleotide hexamer primer, the reactions were carried out at 47°. Stopping the reaction and deactivating the enzyme was perormed at a temperature of 85°. Synthesized cDNA was transferred on ice and stored at -20℃. Primers were designed using the Allel ID software.
Subsequently, reverse transcription-polymerase chain reaction (RT-PCR) was performed, and the collected data was analyzed.
3.4. Gene Expression Buffer Analysis
Gene expression was quantified through quantitative polymerase chain reaction (qPCR), using the Step One Plus Detection System (Applied BiosystemTM, USA). The hypoxanthine phosphoribosyltransferase (HPRT) gene served as the normalization factor and internal control. The 2-∆∆CT comparison method was used to obtain quantitative relative amounts of mRNA.
3.5. Statistical Analysis
Before the therapeutic intervention, parameters, such as age, gender, Body Mass Index (BMI), FKBP5, and SLC6A4, were evaluated using independent t-test and chi-square test. To validate the roles of FKBP5 and SLC6A4 genes in distinguishing MDD patients from HCs, we examined the sensitivity and specificity of these potential biomarkers by calculating the area under the receiver operating characteristic (ROC) curve. The Ct cut-off level for FKBP5 and SLC6A4 was determined as the threshold that yielded the highest sensitivity among the maximum values in the Youden index (sensitivity + specificity - 1). Generally, the Youden index is deemed an effective measure for determining the appropriate cut-off score (20).
An independent t-test was utilized to compare quantitative variables between the two groups, while a paired t-test was used to compare the mean of quantitative variables pre-and post-intervention. In cases where a quantitative variable demonstrated a significant difference between the two groups before the intervention, an analysis of covariance (ANCOVA) test was applied to control for the effects of this difference when comparing the two groups post-intervention. Data analysis was conducted using SPSS version 22, and GraphPad Prism 9 was used for graphing. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Demographic Characteristics
Eighty-four individuals participated in evaluating the sensitivity and specificity of the FKBP5 and SLC6A4 genes and distinguishing between MDD patients (n = 40) and HCs (n = 44). Major depressive disorder patients were divided into the CBT group (n = 20) and the fluoxetine group (n = 20). The mean BDI-II score for patients with MDD was 24.70 ± 2.23. According to the results presented in Table 1, there were no significant differences in age, gender, and BMI between the MDD and HC groups. While the FKBP5 gene expression did not significantly differ between the two groups, the SLC6A4gene expression showed a significant difference.
| Diagnostic Groups | Age (y) | Sex | BMI (kg/m2) | FKBP5 | SLC6A4 | BDI-II Scores |
|---|---|---|---|---|---|---|
| Total | ||||||
| Patients (n = 40) | 31.24 ± 3.88 | 40 (47.6) | 25.04 ± 1.07 | 31.34 ± 2.42 | 35.23 ± 1.90 | 24.70 ± 2.23 |
| Healthy controls (n = 44) | 30.57 ± 3.98 | 44 (52.4) | 25.05 ± 1.12 | 29.22 ± 2.38 | 29.49 ± 4.81 | 8.28 ± 3.75 |
Demographic and Clinical characteristic of MDD and HCs (n = 84) a
4.2. Comparison of the Expression of FK506 Binding Protein 5 and Solute Carrier Family 6 Member 4 Genes for Differentiating the Major Depressive Disorder Group from the Healthy Control Group
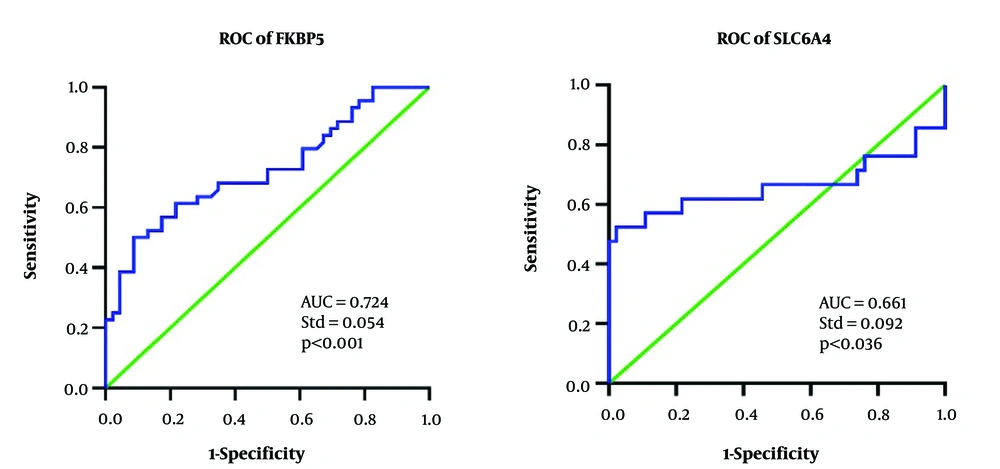
The ROC curve analysis demonstrates that both FKBP5 and SLC6A4 [area under the curve (AUC)] have satisfactory efficiency in distinguishing MDD patients from HCs (Figure 1). For the FKBP5 gene, the AUC was 0.724, with a standard error of 0.054 and an asymptotic 95% confidence interval (CI) ranging from 0.618 to 0.829. For the SLC6A4 gene, the AUC was 0.661, with a standard error of 0.092 and an asymptotic 95% CI ranging from 0.479 to 0.841.
The optimal Ct cut-off points for the genes were found to be 29.27 for the FKBP5 gene and 31.19 for the SLC6A4 gene. In terms of sensitivity and specificity, the FKBP5 gene demonstrated a sensitivity of 68% (95% CI = 0.53 - 0.80) and a specificity of 65%. (95% CI = 0.51 - 0.77). Also, the SLC6A4 gene showed a sensitivity of 61% (95% CI = 0.446 - 0.743) and a specificity of 78%. (95% CI = 0.574 - 0.826). Therefore, both genes exhibited adequate sensitivity and specificity to differentiate between the MDD patients and HCs.
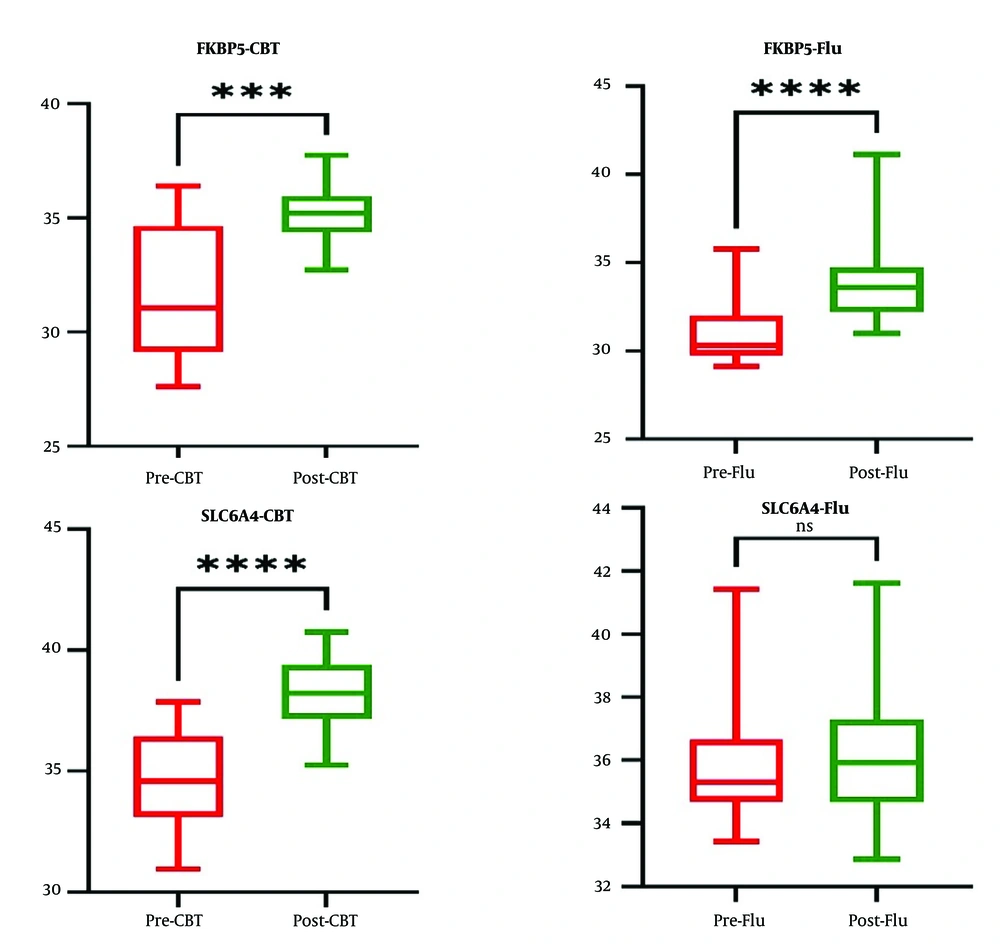
Figure 2 reveals a significant difference in the expression of the FKBP5 gene in both the CBT group and the fluoxetine group before and after the intervention. Before the intervention, the expression of the FKBP5 gene was not significantly different between the CBT and fluoxetine groups. However, the results indicated a significant difference in the expression of the FKBP5 gene after the intervention between the two groups. Notably, the expression of this gene was lower in the CBT group compared to the fluoxetine group.
No significant differences were observed in the expression of the SLC6A4 gene in the fluoxetine therapy group before and after the intervention. However, in the CBT group, a significant difference was observed in the expression of the SLC6A4 gene before and after the intervention. Before the intervention, the expression of the SLC6A4 gene was significantly different between the CBT and fluoxetine groups. To account for this significant difference, an ANCOVA test was employed to compare the gene expression between the two groups. The results revealed a significant difference in the expression of the SLC6A4 gene after the intervention between the two groups. Notably, the expression of this gene was lower in the CBT treatment group compared to the fluoxetine group.
Table 2 illustrates a significant difference in the mean BDI-II scores between the CBT group and the fluoxetine therapy group before and after the intervention. The mean BDI-II score before the intervention was significantly different between the CBT and fluoxetine groups. To account for this significant difference, an ANCOVA test was employed to compare the mean BDI-II scores between the two groups. The results indicated a significant difference in the mean BDI-II scores after the intervention between the two groups, with the mean score being higher in the fluoxetine therapy group. This study focused on patients with MDD of moderate severity and yielded the following findings.
5. Discussion
This study focused on patients with MDD of moderate severity and yielded the following findings.
5.1. Expression Levels of FK506 Binding Protein 5 and Solute Carrier Family 6 Member 4 in Major Depressive Disorder Patients and Healthy Controls
One of the most vital components of the stress response in MDD patients is the reaction of the HPA pathway biomolecules. The FKBP5 biomolecule is one of the principal negative regulators of this axis. This gene negatively regulates the effects of cortisol by inhibiting the interaction between GRs and cortisol (21). A previous study by Hori et al. showed that the expression of FKBP5 was decreased in MDD patients compared to healthy subjects, which is consistent with our research (6). Previous studies support the hypothesis that the diminished expression of the FKBP5 gene not only plays a central role in regulating the function of the glucocorticoid axis but also plays a crucial role in the pathophysiology and phenotype of MDD (22, 23). The HPA axis of MDD patients is hyperactive and releases high levels of glucocorticoid hormones. Glucocorticoid receptors mediate the effects of glucocorticoids in cells; therefore, the higher the levels of glucocorticoids, the more receptors are activated. The FKBP5 gene is responsible for the regulation of GRs in the HPA axis. By increasing the level of glucocorticoids in depressed patients, the expression of the FKBP5 gene will increase in MDD patients to regulate the GR (24).
Serotonin plays an important role in causing depression. Environmental stressors lower serotonin levels through epigenetic changes and decreased serotonin transporter gene expression. Booij et al. showed that the expression levels of the serotonin transporter gene (SLC6A4) in MDD patients are lower compared to the HC group, which is consistent with the present study (25).
Previous research has indicated that alterations in the expression of the SLC6A4gene can lead to brain dysfunction and contribute to the onset of MDD (26, 27). Bakusic et al. showed that SLC6A4 gene methylation in MDD patients is related to HPA axis dysregulation and cortisol reactivity. This supports the hypothesis that the interaction of SLC6A4and HPA is an important component in the pathogenesis of depression (28). The obtained results indicate the role of genetic biomarkers in the diagnosis of MDD from HCs. Therefore, it seems necessary to conduct more research to identify transcriptomic biomarkers for the diagnosis of MDD patients.
5.2. Accuracy of FK506 Binding Protein 5 and Solute Carrier Family 6 Member 4 Genes in Distinguishing Major Depressive Disorder Patients from Healthy Individuals
The neurobiology of MDD remains poorly understood, and no independent and precise theory has been proposed to elucidate the disease pathology (29). A prior study suggested that examining alterations in gene expression could help differentiate various trajectories of mental disorders (30). We demonstrated that the expression levels of the FKBP5 and SLC6A4 genes had acceptable accuracy in distinguishing MDD patients from HCs, which is consistent with the results of previous studies (29, 31). So far, no study has evaluated the sensitivity and specificity of the FKBP5 and SLC6A4 genes in distinguishing MDD patients from HCs. Most existing studies have focused on evaluating changes in gene expression relative to baseline values or in comparison with HCs. Such changes in gene expression can serve as a tool for diagnosing nervous system pathologies (32-34). However, the evaluation of gene expression and related proteins can provide a more accurate differentiation of the disease status (35).
5.3. Changes in the Expression of FK506 Binding Protein 5 and Solute Carrier Family 6 Member 4 Genes in Response to Treatment with Cognitive-Behavioral Therapy and Fluoxetine
Patients with MDD may experience minimal responses to a variety of treatment approaches. Boland et al. showed that genetic responses could help change the course of treatment. Their research identified genes as plausible predictors of treatment responses (36). Decades of research on FKBP5 have demonstrated its role in a variety of psychiatric diseases, potentially serving as a diagnostic and therapeutic target (37). Treatment of MDD patients with antidepressants showed that the BDI-II score decreased and the FKBP5 gene expression increased; this finding is consistent with our study (8, 9). In animal models, the transport of GRs into the nucleus is controlled by FKBP5 by phosphorylating and binding to the chaperone protein. By changing the phosphorylation of GRs, antidepressants modulate the expression of FKBP5 and GRs gene, thus improving the clinical symptoms of depression (38). Clinical studies have highlighted the FKBP5 gene as a biomarker of treatment response. The change in FKBP5 gene expression is a promising target for evaluating treatment response (37). Alteration of FKBP5 expression by antidepressants can serve as a predictive biomarker for treatment response. The findings of this study reveal that FKBP5 gene expression is a reliable biomarker in response to fluoxetine. Molecular evidence has demonstrated that CBT plays a significant role in DNA methylation and the alleviation of clinical symptoms in psychiatric disorders (39). The neurobiological effects of psychotherapy are linked to the establishment of neural balance, neuroendocrine regulation, and reduction of clinical symptoms in patients with MDD (40, 41).
In the current study, no changes were observed in the level of SLC6A4 gene expression after treatment with the antidepressant fluoxetine.
According to Kao et al., in MDD patients treated with antidepressants, there is a significant relationship among treatment duration, reduced depression score, and SLC6A4 mRNA expression decrease (42).
Some abundant long non-coding RNAs (lncRNAs) influence gene expression by functioning as competitive endogenous RNAs. They bind to the homeobox binding sites through microRNAs (MiRs), thereby suppressing and downregulating gene expression (43, 44).
Following 12 consecutive sessions of CBT, a significant decrease was observed in the expression level of SLC6A4 post-treatment. A systematic study conducted by Pellicano et al. revealed that changes in gene methylation occurred in patients who respond positively to CBT. These findings suggest that psychotherapy is associated with dynamic alterations in the epigenetic mechanisms (45). The exact mechanism by which CBT impacts the nervous system is not yet fully understood. However, it has been shown that CBT induces changes in various brain areas, primarily by modulating the functions of cognitive, emotional, and emotional regulation networks (46). Moreover, as reported by Uscinska et al., psychotherapy triggers changes in the structure and function of the brain, improves neural circuits, promoting plasticity within the nervous system by influencing the cerebral cortex (47).
5.4. Limitations and Strengths
While this study had several strengths, such as comparing MDD patients with a healthy group in assessing sensitivity and specificity in identifying patients from HCs, and response to treatment with CBT and fluoxetine therapy, it also had several limitations. These limitations included a small sample size, a short follow-up period of patients, the use of single-dose antidepressant medication, and a lack of examination of other biomarkers, epigenetic factors, and mRNA-related proteins.
5.5. Conclusions
The findings of this study reveal that FKBP5 and SLC6A4 biomarkers, associated with the HPA axis and serotonergic pathway respectively, are reliable indicators in distinguishing MDD patients from HCs. These biomarkers can serve as complementary tests for identifying individuals with depression. Furthermore, CBT has been validated as an effective treatment method for MDD patients exhibiting moderate depression scores. This therapeutic approach is associated with a decrease in the BDI-II scores and a decrease in the expression of FKBP5 and SLC6A4 genes. In MDD patients treated with fluoxetine, no change was observed in the SLC6A4 gene expression; however, a decrease in FKBP5 gene expression was noted. This study suggests that the FKBP5 biomarker is an appropriate candidate for initial screening of MDD patients with mild to moderate severity and for evaluating treatment responses. Future research should aim to evaluate a larger sample size, extend the follow-up period, and assess additional molecular markers.