1. Background
Generalized anxiety disorder (GAD) is a widespread mental health condition among adults, characterized by persistent and uncontrollable worry about various situations or activities. Symptoms typically include fatigue, difficulty concentrating, restlessness, irritability, sleep disturbances, and physical complaints (1). Additionally, individuals with GAD are twice as likely to use emergency departments compared to those with other major psychiatric disorders (2). Research on this disorder has gained attention due to its high prevalence and the frequent impact it has on daily life (3, 4).
The current treatment guidelines for anxiety disorders include a variety of both medication-based and therapeutic interventions (5). Despite the availability of evidence-based psychotherapies, many patients with anxiety disorders lack access to these treatment options. This may be due to factors such as a shortage of trained therapists, physicians’ hesitancy to recommend evidence-based therapies, or limited funding for non-pharmacological options. Additionally, psychotropic medications often produce inconsistent results and may lead to unwanted side effects (6). In general, although numerous treatment options are available, complete recovery is rarely attained, highlighting the need to explore new therapeutic alternatives for individuals with anxiety disorders (7, 8). Furthermore, an essential and current comparison of various treatments for GAD is necessary, given their high costs and often unsatisfactory results (9).
Electroencephalographic (EEG) neurofeedback is a neuromodulation technique in which individuals learn to control their brain activity through operant conditioning, with the goal of enhancing various cognitive functions (10). The EEG neurofeedback training has been shown to alter long-term neural activity and connectivity, helping to regulate symptoms such as excessive behavioral and physiological arousal, a key feature of anxiety disorders (7, 8, 11). It is known that different EEG patterns reflect a person's level of arousal or relaxation by measuring the electrical oscillations of neuronal activity in the cerebral cortex (12).
The alpha rhythm (8 - 12 Hz) was the first oscillation discovered, detectable during relaxed wakefulness and increasing with eye closure (13). Sensorimotor rhythm (SMR) oscillations, ranging from 12 - 15 Hz, can be observed over the sensorimotor cortex during states of relaxed wakefulness and decreased motor activity, with an associated increase in amplitude (14, 15). Furthermore, literature indicates a reliable increase in theta oscillations during meditation practice, regardless of the specific type of meditation or the amount of training, in addition to alpha waves (16, 17).
Studies on EEG neurofeedback and its effects on GAD have demonstrated its effectiveness in reducing symptoms. Rice et al. conducted one of the earliest studies in this area with 38 students and staff from SUNY-Albany. Participants underwent eight sessions (twice per week for four weeks) of either frontal electromyographic (EMG) biofeedback, EEG neurofeedback designed to enhance or reduce alpha rhythm, or a control condition. All treatment groups exhibited significant decreases in State-Trait Anxiety Inventory (STAI)-trait anxiety and psychophysiological symptoms, as measured by the psychosomatic symptom checklist. Notably, only the group receiving alpha-increase biofeedback showed a marked reduction in heart rate reactivity to stressors during a separate testing session, with the decrease in self-reported anxiety persisting for six weeks post-treatment (18). A more recent study by Hou et al. investigated the effectiveness of EEG neurofeedback training targeting alpha activity over the parietal lobe in individuals with GAD. In this study, 26 female patients were randomly assigned to either a left parietal lobe training group (P3) or a right parietal lobe training group (P4) and underwent ten 40-minute sessions of alpha-increase training in the designated areas. Both groups showed significant decreases in STAI-S scores two weeks after the fifth session, with further reductions at the four-week follow-up. Additionally, compared to baseline, scores on the STAI-T, Beck Depression Inventory (BDI-II), and Insomnia Severity Index (ISI) were reduced at the two-week point, and these improvements persisted at the four-week follow-up, with no significant differences between the P3 and P4 groups (19). In a study by Dadashi et al., the effects of increasing alpha and theta brain wave amplitudes in the occipital region (O1 and O2) on GAD symptom severity were examined. Twenty-eight patients referred to psychiatric and clinical psychology centers were divided into two groups: Fourteen received neurofeedback treatment (15 sessions of 30-minute alpha training and 15 sessions of 30-minute theta training), and 14 were placed on a waiting list. Assessments using the GAD Scale (GAD-7) and the global Assessment of Functioning (GAF) Scale indicated significant improvements in global functioning and a reduction in GAD symptoms in the treatment group compared to controls (20).
Based on the studies mentioned and building on the theoretical framework provided by Gruzelier et al. (21) and others, the parietal regions appear to play a critical role in modulating neural networks involved in relaxation and anxiety reduction. Specifically, increasing alpha and theta activity in the parietal areas (using a Pz electrode placement) is thought to facilitate a hypnagogic state, promote long-distance neural connectivity, and enhance emotional regulation. In contrast, SMR training was applied over Cz because research has demonstrated that enhancing SMR (12 - 15 Hz) in the sensorimotor region can improve attentional control and reduce anxiety through the modulation of motor and cognitive processes (22, 23).
2. Objectives
The goal of this study was to compare the effectiveness of two neurofeedback protocols: One aimed at increasing SMR amplitude over Cz and the other focused on enhancing alpha-theta (AT) amplitude over Pz. While both protocols have been individually explored in previous research, direct comparisons remain limited. By investigating the differential effects of these approaches on anxiety symptoms, this study aims to provide a clearer understanding of their relative efficacy and underlying mechanisms. This comparison contributes to the refinement of neurofeedback interventions, offering evidence-based guidance on selecting the most effective protocol for individuals with anxiety disorders. Additionally, these findings may help optimize personalized treatment strategies by identifying the specific neural mechanisms targeted by each protocol.
3. Methods
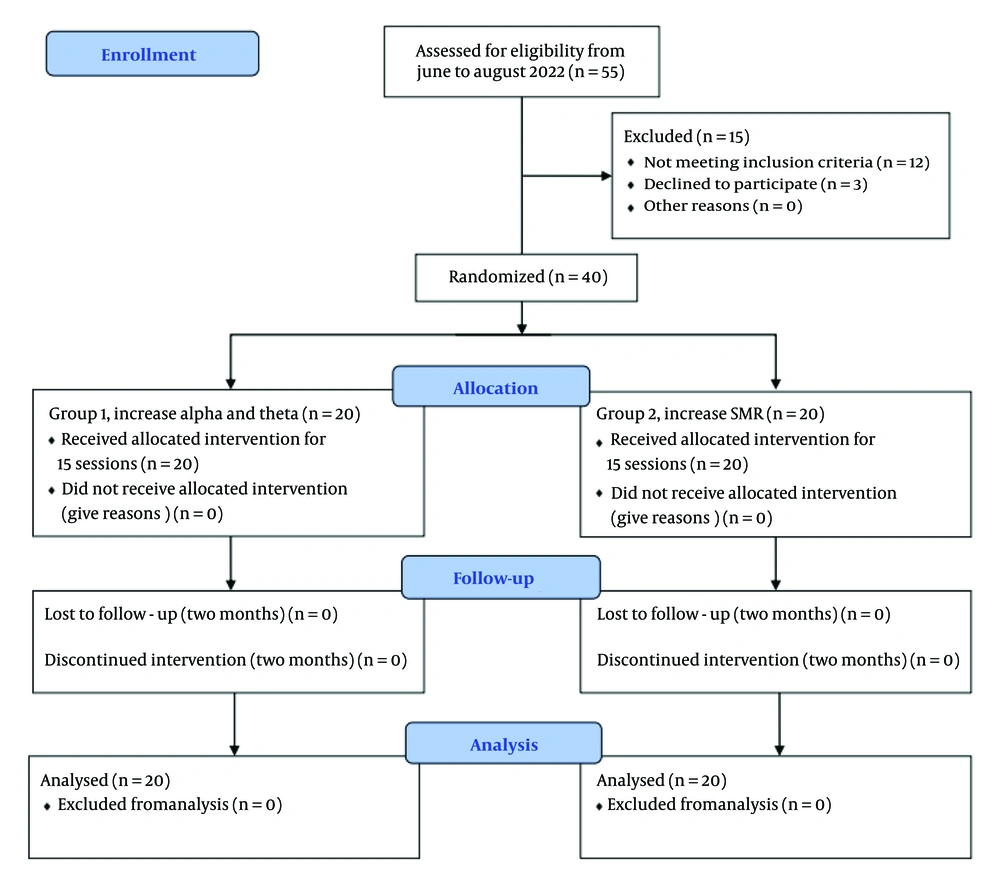
This study was a single-center, single-blinded, parallel-design trial with two experimental groups, conducted between June 2022 and July 2023. Participants were recruited from a hospital in Tehran, Iran. Written informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1401.161) and was registered on ClinicalTrials.gov (NCT06361953). The flowchart is presented in Figure 1.
3.1. Participants
To determine the sample size using G*Power, for the ANOVA test, we selected a moderate effect size of 0.4 as a reasonable assumption in cases where meaningful but not extreme changes in outcomes are expected, an error of 0.05, and a minimum acceptable statistical power of 0.8, due to the limited number of samples available. Based on these criteria, the sample size in each group should be at least 18. The research focused on university students diagnosed with GAD, who were treated at the outpatient psychology clinic of a hospital located in Tehran, Iran, primarily because they were readily available and provided a relatively homogeneous sample in terms of educational background and cognitive functioning, which helps control for extraneous variables. A total of 40 individuals, with 20 in each group, willingly volunteered to participate. After assigning ID numbers to all potential participants, we used a random generator app to randomly assign them to either group one or two. A standard psychiatric interview conducted by a psychiatrist was used to diagnose participants based on the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). The researchers informed the participants about the study's purpose but did not disclose the differing protocols used in the two groups.
The inclusion criteria required participants to have normal hearing and vision, no history of EEG neurofeedback or any other therapy in the past year, and a diagnosis of GAD based on standard psychiatric interviews. They had to be 20 years or older, with no history of neurological disorders or ongoing treatments for other conditions. The exclusion criteria ruled out individuals receiving any other form of treatment or those who missed more than one session, to ensure that the observed effects were solely attributable to neurofeedback.
3.2. Procedure
This study was conducted at the hospital’s community counseling center. To ensure participant privacy during services, a dedicated room was assigned for all EEG neurofeedback training sessions. Psychological assessments were conducted in a separate counseling room to maintain confidentiality. Participants in both groups attended 15 half-hour training sessions over five weeks, with three sessions per week and a one-day gap between each session. During each session, participants were seated in front of a monitor and shown videos of natural scenery. The neurofeedback system was programmed so that when their brainwave activity increased beyond the expected threshold, the video would continue playing as a reward. If the required brainwave increase was not achieved, the video would pause. The feedback was exclusively visual, without any auditory reinforcement (24).
The ProComp system was used with three sensors applied using neuroconductor gel. In both protocols, only one active electrode was used. The reference electrode was placed on the left earlobe, and the ground electrode on the right earlobe. The active electrode for Group 1 was positioned at Pz, while for Group 2, it was placed at Cz, following the international 10 - 20 system.
Group 1 (AT increase protocol): Participants received 15 sessions aimed at increasing alpha (8 - 12 Hz) and theta (4 - 8 Hz) power. The primary goal of this protocol was to facilitate a gradual transition in which theta power exceeded alpha power during the session — referred to as the "crossover effect". This transition has been linked to a hypnagogic state, characterized by enhanced creativity and reduced anxiety (25, 26).
Group 2 (SMR enhancement protocol): Participants received 15 sessions targeting SMR (SMR, 12 - 15 Hz) enhancement. Sensorimotor rhythm training is associated with improved cognitive control and reduced motor-related anxiety (24).
To ensure high-quality EEG data, real-time artifact detection and rejection techniques were implemented. The system continuously monitored for eye blinks, muscle movement, and excessive signal noise. A low-pass filter with a 60-microvolt threshold was applied to exclude high-amplitude artifacts (27).
Before the first session, a 2-minute resting-state EEG recording was taken with eyes closed. These baseline recordings were used as a reference to adjust individual feedback thresholds dynamically, ensuring that training progress was measured relative to each participant’s resting state (21, 24).
The anxiety levels of the patients were assessed three times: Before the beginning of the sessions (pre-test), after the end of the sessions (post-test), and two months after the last session (follow-up). Scales included the Beck Anxiety Inventory (BAI), the Perceived Stress Questionnaire, and Spielberger's STAI. The BAI, Perceived Stress Scale (PSS), and STAI were selected for their well-established validity and reliability in assessing anxiety-related symptoms. The BAI is widely used to measure the severity of anxiety symptoms, making it highly relevant for evaluating treatment effects in GAD (28). The PSS assesses the psychological impact of stress, which is closely linked to anxiety disorders, providing insight into stress-related symptom changes (29). The STAI differentiates between temporary (state) and chronic (trait) anxiety, allowing for a more nuanced understanding of how neurofeedback interventions impact different aspects of anxiety in individuals with GAD (30).
3.3. Scales
The BAI consists of 21 items, each rated on a four-point Likert scale ranging from "Not at all" to "Severely". The responses reflect symptoms experienced over the past month, including the day of assessment (31). The BAI contains many items that address somatic symptoms of anxiety, such as wobbliness in one's legs, feeling dizzy or lightheaded, feeling unsteady, and difficulty breathing. The items are scored from 0 to 3, with a total range of 0 - 63. Scores from 0 - 7 are considered minimal, 8 - 15 are mild, 16 - 25 are moderate, and 26 - 63 are severe (32). In this study, the Persian version was used, which showed good reliability (0.72), very good validity (r = 0.83), and excellent internal consistency (alpha = 0.92) (33).
The PSS, developed in 1983 by Cohen et al., is a 14-item questionnaire designed to assess an individual's perceived stress over the past month. It evaluates their thoughts, emotions, and perceived ability to manage stressful events, as well as their coping strategies. The PSS measures both psychological pressure and stress levels experienced by the person. Furthermore, it can help identify risk factors for behavioral disorders and highlight the impact of stressful relationships. A higher score indicates a greater level of perceived stress. The scale has a maximum score of 40 points, with higher scores reflecting higher levels of perceived stress. Scores between 0 and 13 are categorized as low stress, scores from 14 to 26 as moderate stress, and scores from 27 to 40 as high perceived stress [as cited in (34)]. In this study, the Persian version of the scale was used, which demonstrated good reliability with a coefficient of 0.84 (35).
Spielberger's STAI measures two types of anxiety: State anxiety and trait anxiety. The test consists of 40 questions, with 20 questions for each type. Participants are asked to report their current feelings for the state anxiety scale and their general, long-term feelings for the trait anxiety scale. Each item is rated on a 4-point Likert scale (ranging from 0 to 3 points). The assessment is divided into two subscales, each containing 20 items, and the total score is derived from both subscales (36). In this study, the Persian version of the STAI was used, which demonstrated strong internal consistency with Cronbach's alpha values of 0.886 for trait anxiety and 0.846 for state anxiety. The convergent validity between the STAI-Y and the BAI was found to be 0.612 for trait anxiety and 0.643 for state anxiety (37).
3.4. Statistical Analysis
Descriptive statistics were utilized to present data, including mean ± SD for numerical data and numbers for categorical data. The normality of the data was assessed using the Shapiro-Wilk test. To compare demographic data differences between groups, the chi-square test and t-test were employed. The repeated measures ANOVA test was used to compare mean scores in the pretest, posttest, and follow-up. For comparing the two protocols, a mixed-design ANOVA test was used. All analyses were conducted using IBM SPSS software version 26 with a 95% confidence interval.
4. Results
A total of 20 subjects were included in each group for analysis. Twenty-three participants were female, and 17 were male. Of the total, 14 participants (35%) held master's degrees, while 26 (65%) held PhDs. Demographic information by group is summarized in Table 1. There was no significant difference in the distribution of gender (χ2 = 2.55, P = 0.11) and education (χ2 = 0.44, P = 0.51) between groups. The independent t-test between the mean age of group one (29.45 ± 5.30) and group two (30.70 ± 5.32) showed no significant differences (t = -0.98, P = 0.32). The mean and standard deviation (SD) of variables is summarized in Table 2.
| Variables | AT Group | SMR Group |
|---|---|---|
| Age | 29.60 ± 1.81 | 30.55 ± 5.38 |
| Gender (female) | 8 (40) | 15 (75) |
| Education (PhD) | 11 (55) | 15 (75) |
Abbreviations: SMR, sensorimotor rhythm; AT, alpha-theta.
a Values are expressed as No. (%) or mean ± SD.
| Variables | AT (N = 20) | SMR (N = 20) | ||||
|---|---|---|---|---|---|---|
| Pre-test | Post-test | Follow-up | Pre-test | Post-test | Follow-up | |
| BAI | 19.50 ± 8.58 | 7.30 ± 7.54 | 7.90 ± 7.22 | 24.80 ± 8.80 | 14.95 ± 8.48 | 15.85 ± 8.15 |
| State | 59.75 ± 3.27 | 51.20 ± 4.43 | 52.55 ± 4.87 | 61.90 ± 4.51 | 49.00 ± 6.96 | 50.00 ± 6.68 |
| Trait | 61.70 ± 5.15 | 54.90 ± 5.03 | 55.80 ± 4.88 | 62.75 ± 5.46 | 53.00 ± 6.96 | 54.05 ± 7.47 |
| PSS | 16.60 ± 2.81 | 10.05 ± 2.43 | 20.50 ± 4.82 | 18.70 ± 2.31 | 12.65 ± 2.41 | 23.55 ± 4.57 |
Abbreviations: SMR, sensorimotor rhythm; BAI, Beck Anxiety Inventory; PSS, Perceived Stress Scale; AT, alpha-theta.
a Values are expressed as mean ± SD.
4.1. Training Effect for Each Group
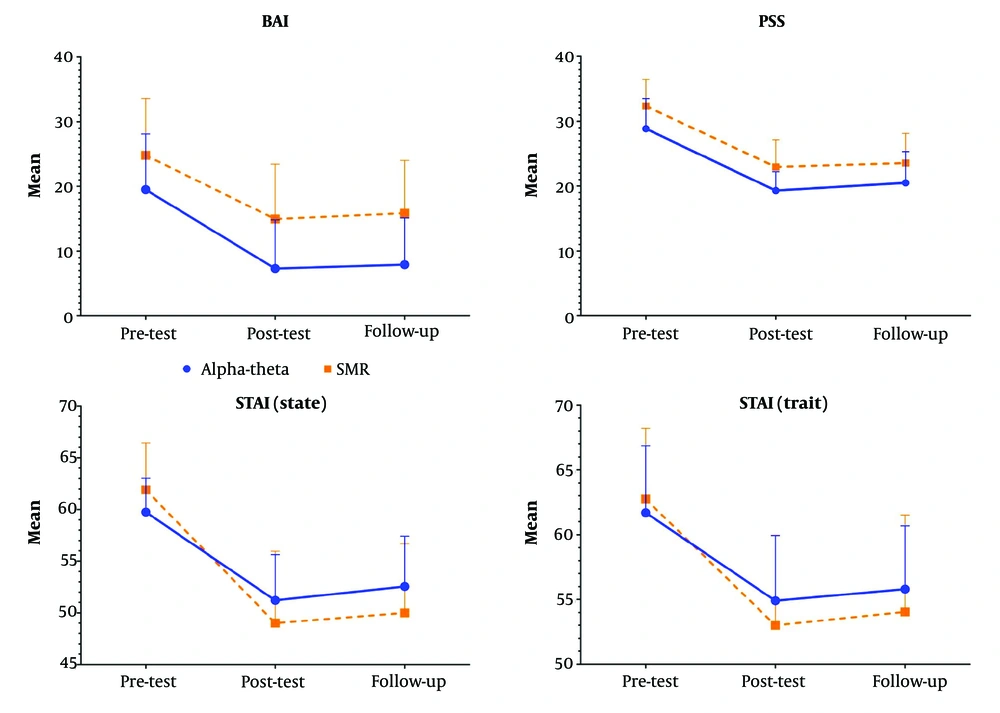
The mean ± SD of each group was summarized in Table 2. A repeated measures ANOVA was used to compare training effectiveness. Mauchly's test revealed a violation of the assumption of sphericity [χ2 (2) = 23.78, P = 0.01] for BAI, leading to using a Huynh-Feldt correction. There was statistically significant change between pretest, posttest and follow up in AT group for BAI (F = 110.39, P = 0.01, Np2 = 0.85, ε = 0.59), pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) not posttest and follow up (P = 0.31), For STAI (state) (F = 49.62, P = 0.01, Np2 = 0.72, ε = 0.79) pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) not posttest and follow up (P = 0.10), for STAI (trait) (F = 36.30, P = 0.01, Np2 = 0.65, ε = 0.71) pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) not posttest and follow up (P = 0.23), and PSS (F = 51.13, P = 0.01, Np2 = 0.72, ε = 0.70) pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) not posttest and follow up (P = 0.17).
For SMR group in BAI (F = 93.74, P = 0.01, Np2 = 0.83, ε = 0.60) pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) not posttest and follow up (P = 0.08), for STAI (state) (F = 73.50, P = 0.01, Np2 = 0.79, ε = 0.61) pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) and posttest and follow up (P = 0.02), for STAI (trait) (F = 22.57, P = 0.01, Np2 = 0.54, ε = 0.55) pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) not posttest and follow up (P = 0.38) and PSS (F = 175.00, P = 0.01, Np2 = 0.90, ε = 0.62) pairwise comparison shows the significant difference was between pre-test with posttest (P = 0.01) and follow up (P = 0.01) not posttest and follow up (P = 0.057).
4.1.1. Comparing Two Groups
A mixed-design ANOVA with Greenhouse-Geisser correction for BAI revealed a significant main effect of time, F = 203.81, P < 0.001, partial η2 = 0.84, and a significant main effect of group, F = 7.89, P = .008, partial η2 = 0.17. The time × group interaction was not significant (P = 0.09).
For state anxiety, a significant main effect of time was revealed, F = 122.87, P < 0.001, partial η2 = 0.76, and a nonsignificant main effect of group, F = 0.36, P = 0.54, partial η2 = 0.10. The time × group interaction was significant (P = 0.01).
For trait anxiety, a significant main effect of time was revealed, F = 49.74, P < 0.001, partial η2 = 0.56, and a nonsignificant main effect of group, F = 0.31, P = 0.57, partial η2 = 0.008. The time × group interaction was not significant (P = 0.20).
For PSS, a significant main effect of time was revealed, F = 159.14, P < 0.001, partial η2 = 0.80, and a significant main effect of group, F = 8.52, P = 0.006, partial η2 = 0.18. The time × group interaction was not significant (P = 0.77) (Figure 2).
5. Discussion
In this study, we evaluated the effectiveness of two EEG neurofeedback protocols (AT and SMR) in reducing anxiety symptoms and perceived stress among individuals with GAD. Participants underwent 15 sessions of either the AT protocol at Pz or the SMR protocol at Cz. The results demonstrated a significant reduction over time in BAI, state anxiety, trait anxiety, and PSS scores, confirming the overall efficacy of both neurofeedback protocols in alleviating anxiety symptoms. For state anxiety, there was a significant time × group interaction, implying that the reduction patterns differed between the two protocols. Although both groups experienced a decrease in state anxiety over time, the SMR group showed a slightly greater immediate reduction in the post-test compared to the AT group, suggesting that SMR training may have a more immediate impact on momentary anxiety states.
Regarding trait anxiety, both groups exhibited a significant reduction over time, but no significant group differences or interaction effects were observed, suggesting that both protocols were equally effective in reducing long-term anxiety traits. These findings suggest that while both neurofeedback protocols effectively reduced anxiety symptoms and perceived stress, the SMR protocol might provide more immediate relief for state anxiety. Future studies incorporating objective neurophysiological markers are needed to further validate these findings and explore the underlying mechanisms of each protocol.
Results indicated that the AT protocol significantly reduced anxiety levels. These findings align with Dadashi et al.'s research, which demonstrated that enhancing alpha waves can alleviate symptoms of GAD (20). After EEG neurofeedback training to boost AT activity, individuals with GAD experienced expected decreases in PSS, STAI, and BAI scores. Notably, our study observed a similar pattern in the reduction of BAI scores across both protocols. However, more detailed investigations using objective tools are needed to assess brain activity following each protocol and to better understand the specific effects of each protocol on brain function.
Anxiety disorders are frequently triggered and maintained by biases in processing threat-related information. This suggests that the attention system of anxious individuals is more sensitive to threat-related than to neutral stimuli. These biases play a crucial role in the onset and continuation of anxiety disorders like GAD (19, 38). Mansell's top-down model of processing biases in anxiety suggests that the anterior cingulate cortex, lateral prefrontal cortex, and parietal cortex play key roles in regulating attention (39).
Recent functional magnetic resonance imaging (fMRI) studies have identified three distinct attentional networks. The first is the alerting network, which is activated in the frontoparietal cortex and thalamus. The second is the orienting network, characterized by high activity in the superior parietal region and temporal-parietal junction, with a bias towards the right hemisphere. Finally, the executive control network is activated in the anterior cingulate and both right and left frontal areas (40).
Building on this model and its findings, Hou et al. demonstrated the effectiveness of EEG neurofeedback training targeting the parietal lobe in enhancing attention control (19, 41), and our findings support the efficacy of this protocol in GAD patients. However, since we did not conduct a biological evaluation in our study, we cannot be certain whether the results obtained are due to changes in the activity of brain networks or other factors. This statement should help to clarify the limitations of our study for the academic journal.
Group two, undergoing the SMR protocol, demonstrated a significant decrease in BAI, PSS, and STAI scores. The SMR training regimen is known to improve attention (42). This occurs when an individual is still yet alert, supported by brain activity in central scalp regions (43, 44). Research has validated the efficacy of a 12 - 15 Hz frequency range in anxiety management (23). Nonetheless, its extended duration, delayed impact, and variability among individuals limit its clinical application (22, 45, 46).
The SMR EEG neurofeedback has proven effective in treating children with attention-deficit hyperactivity disorder (ADHD), and it has also shown positive effects on the attentional performance of healthy individuals (25, 47, 48). One possible explanation for the effectiveness of SMR EEG neurofeedback training is that it functions as a bottom-up mechanism within the thalamic-cortical circuitry, enhancing its inhibitory processes. By increasing SMR, this training may boost the brain's ability to filter out irrelevant sensory information, leading to improved somatosensory processing (25, 43).
Moreover, SMR training may stabilize vigilance by regulating the locus coeruleus noradrenergic system. Its activation has been demonstrated to affect the sleep spindle circuit (49). Research has shown that engaging in attention-focused practices, such as yoga and meditation, can be highly effective in reducing symptoms of anxiety (50, 51). In addition, in clinical settings, lack of focus is a common anxiety symptom. Therefore, it was hypothesized that improving attention-related SMR activity could alleviate anxiety (52). Our study supports the effectiveness of this protocol for anxiety, but further research is necessary to assess its impact on brain function.
The SMR training appears to enhance cortical regulation and promote a state of focused calm. This mechanism likely leads to more immediate reductions in physiological arousal and, consequently, state anxiety. In contrast, the AT protocol — designed to facilitate a hypnagogic state and promote long-term mood and creativity changes — may not be as effective for rapid anxiety relief. The more direct modulation of arousal-related neural circuits via SMR training might therefore explain its superiority in alleviating state anxiety during the intervention (25, 27, 53).
It should be noted that this study had some limitations. Only university students were recruited for this study, allowing for a more homogeneous sample and controlled variables. However, this may limit the generalizability of our findings to a broader population with more diverse demographic and clinical characteristics. The EEG neurofeedback training usually involves more sessions; however, our participants could not undergo additional sessions because neurofeedback is not considered a first-line treatment. Despite obtaining informed consent from the participants, hospital policy restricted us from enrolling participants in longer training. Increasing the number of sessions could potentially reduce the differences between the two protocols.
Additionally, a significant limitation of this study was the inability to include a placebo group, as we could not exclude patients referred to the treatment center, which made it challenging to establish a proper control group. The lack of a placebo group may have impacted the ability to fully isolate the effects of the treatment from other factors. Furthermore, this study relied solely on subjective data, which can be influenced by participant biases and perceptions.
Future research should incorporate objective data collection methods, such as EEG analysis, to provide a more comprehensive understanding of the effectiveness of different neurofeedback protocols. Using objective measures will not only enhance the reliability of the findings but also contribute to more robust conclusions. Prioritizing objective data will significantly advance the field, improve the validity of research outcomes, and better inform treatment strategies.
5.1. Conclusions
The results indicated that both the AT and SMR EEG neurofeedback protocols were effective in reducing GAD symptoms. Notably, the SMR protocol was superior in reducing state anxiety, while improvements in other variables were similar between the two protocols. Given the high treatment costs associated with neurofeedback, understanding the distinct effects of each protocol may help optimize cost-effectiveness and enhance treatment outcomes by guiding the selection of the most appropriate approach based on individual symptom profiles.
In clinical practice, these findings suggest that SMR neurofeedback may be particularly beneficial for individuals experiencing situational or performance-related anxiety, while both protocols remain viable options for broader anxiety symptom reduction. Future studies should explore the integration of neurofeedback with other therapeutic modalities and incorporate objective measures such as neuroimaging, heart rate variability, and other physiological markers to further elucidate the neural mechanisms underlying the efficacy of these protocols and validate these results.