1. Background
The global prevalence of diabetes is estimated to be 9.3% (463 million people) in 2019, rising to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045. Type 2 diabetes mellitus (T2DM) accounts for approximately 90% of all diabetes cases and presents significant health challenges worldwide (1). Substantial evidence indicates an elevated risk of Alzheimer’s disease (AD) in individuals with T2DM compared with that in non-diabetic subjects. Despite extensive research, the precise molecular mechanisms underlying the association between T2DM and AD remain incompletely understood. Nevertheless, shared pathogenic factors, such as aberrant protein processing, dysregulation of insulin signaling, oxidative stress, and chronic inflammation, are implicated. Cognitive decline in T2DM intensifies challenges in disease management, resulting in poor glycemic control, increased insulin resistance, and enhanced oxidative stress. A direct correlation between hyperglycemia and dementia risk further emphasizes the significance of understanding the T2DM-AD nexus (2).
The T2DM, characterized by persistent hyperglycemia, significantly elevates the risk of AD, a neurodegenerative disorder marked by cognitive decline and memory impairment. Chronic neuroinflammation, a hallmark of AD pathology, is primarily mediated by microglia. In AD, these cells undergo pathological activation, releasing neurotoxic factors that exacerbate neuronal injury and cognitive decline (3). The key players in this process include the soluble triggering receptor expressed on myeloid cells 2 (sTREM2)-apolipoprotein E (APOE) signaling pathway, which regulates microglial metabolic activity and is crucial for amyloid-β (Aβ) clearance (4, 5). Notably, the APOE4 isoform, a genetic risk factor for AD, impairs microglial modulation and Aβ mitigation (3). Furthermore, TYRO protein tyrosine kinase-binding protein (TYROBP), a microglial activity regulator, has emerged as a potential therapeutic target due to its ability to suppress pro-inflammatory cytokines and enhance Aβ phagocytosis (6).
The T2DM and AD are age-associated conditions that are increasingly prevalent. Several studies have demonstrated that patients with T2DM are at a significantly higher risk of cognitive decline and impairment than healthy individuals (7). Understanding their complex interplay is critical. While there is no cure for AD, lifestyle interventions, including physical activity, have shown promise in mitigating cognitive decline. Aerobic exercise (TPMP), specifically, is recognized for enhancing cognitive performance (8).
The human body demonstrates a robust circadian rhythm, governed by a hierarchical network of endogenous oscillators. A central pacemaker, located within the suprachiasmatic nucleus (SCN) of the hypothalamus, functions as the master clock, coordinating the activity of peripheral oscillators situated within metabolic tissues. This complex system ensures the temporal alignment of diverse metabolic pathways with the 24-hour light-dark cycle. Entrainment of these oscillators is achieved through external zeitgebers, including photic cues, nutritional intake, and physical activity, thereby maintaining synchrony with the external environment (9).
Circadian rhythms orchestrate a sophisticated bidirectional regulatory network influencing metabolic and inflammatory pathways, presenting significant implications for disease prevention and chronotherapeutic strategies. The molecular clock intrinsically regulates a substantial portion of the metabolome, with dietary entrainment via nutrient-sensing pathways and chromatin remodeling further shaping the metabolic flux, notably in glucose and lipid processing (10). Concurrently, immune cells possess autonomous circadian oscillators that modulate inflammatory responses, including TLR signaling, cytokine secretion, and macrophage polarization. Rhythmic expression of key inflammatory regulators, such as TLRs and NF-κB, underscores the temporal sensitivity of immune responses and their clinical relevance in conditions such as sepsis and arthritis (11).
Perturbations to this intricate system, often arising from contemporary lifestyle factors, contribute to metabolic syndrome, chronic inflammation, and age-related comorbidities through mechanisms including CLOCK:BMAL1 dysregulation and NAD+/Sirt1 dysfunction (9). Conversely, interventions such as chronoexercise, time-restricted feeding, and circadian immunotherapy leverage the temporal organization of these pathways to optimize therapeutic outcomes. Critical crosstalk nodes, including SIRT1, REV-ERBα, and the gut microbiome, serve as pivotal links between circadian clocks and metabolic/inflammatory processes, highlighting the potential for targeted interventions to restore rhythmic homeostasis (12, 13).
Moreover, studies have consistently shown that exercise and physical activity positively impact working memory, thereby significantly strengthening it. Aerobic exercise, in particular, has been found to increase the efficiency of working memory by enhancing the activity of the prefrontal cortex (14). Therefore, the effectiveness of exercise on selective cognition is likely dependent on the nature of the cognitive functions involved and the brain substrates related to them (15). Despite ongoing debate, the mechanisms underlying the beneficial effects of exercise on memory remain unclear.
Beyond the effect of exercise, the role of time of day as an essential factor that can affect the beneficial effects of exercise on cellular metabolism and improving glycemic control has been highly regarded. The two phases exhibit distinct metabolic patterns due to the combined effects of light exposure, nutritional intake, and cellular activity. The signals that regulate the biological clock, known as Zeitgebers, are believed to play a role in modulating the metabolic impact of exercise (16). The timing of physical exercise can significantly influence how our bodies manage blood sugar. Therefore, carefully planning when to exercise could be a valuable strategy for preventing and controlling type 2 diabetes. Studies indicate that exercising in the afternoon, compared to the morning, often leads to better blood sugar regulation, as measured by continuous glucose monitoring (CGM), and improved insulin sensitivity (17). This might be because our muscles are more efficient at using glucose and exhibit a higher oxidative capacity during afternoon or evening workouts compared to morning sessions (18). However, research findings are mixed; some studies do not show any significant impact of exercise timing on blood sugar control. It’s possible that more intense or longer workouts are needed to see substantial changes in our body's daily rhythms and glucose metabolism (19). Furthermore, endurance exercise capacity has been shown to vary throughout the day, with significant differences observed between early evening and early morning (20).
2. Objectives
Despite these findings, the optimal time of day for exercise that maximizes its beneficial effects remains unclear. Exercises performed at different times of the day yield different outcomes. Therefore, this research aimed to address the following question: Which exercise time — morning or evening — provides greater health benefits for elderly women with T2DM, particularly regarding key health indicators?
3. Methods
3.1. Study Design
The current research was applied with a quantitative approach and utilized a semi-experimental design with a pre-test-post-test framework, including a control group.
3.2. Participants and Randomization
This study enrolled 45 women, aged 55 to 65 years, all diagnosed with T2DM. Participants were residents of Chaharmahal and Bakhtiari provinces and had a documented medical history at one of the comprehensive urban health service centers in Kiyar city. Accounting for attrition, a total of 15 participants per group were examined (21, 22). To ensure unbiased allocation to study groups, participants were randomly assigned to different intervention groups using simple randomization. This study employed a parallel-group randomized controlled trial design with a 1:1:1 allocation ratio. The inclusion and exclusion criteria for participants in the study are listed in Table 1.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Diagnosed with T2DM for at least five years | Injury or inability to perform exercise programs |
| Age range of 60 - 65 years old | Neurological disorders affecting mobility or cognition |
| Type 2 diabetes controlled with oral medications such as metformin (no additional medications) | History of serious cardiovascular diseases, advanced renal disease, active cancer, or uncontrolled thyroid disorders |
| A sedentary lifestyle is defined as less than 150 minutes of moderate-intensity aerobic exercise per week for at least 6 months prior to the study. | Acute or chronic inflammatory conditions |
| Ability to participate in exercise programs | Inability to provide informed consent |
Abbreviation: T2DM, type 2 diabetes mellitus.
3.3. Blinding
To mitigate bias in outcome assessment, a single-blind approach was implemented. Specifically, the outcome assessors were blinded to the participants' treatment group assignments. This was achieved through a data coding procedure wherein all participant data, including questionnaire responses and laboratory results, were anonymized and assigned a unique numerical code prior to assessment and the commencement of the exercise protocol. The assessors received only the coded data, devoid of any information pertaining to the participants' group assignments.
3.4. Recruitment and Consent
Inclusion criteria encompass a confirmed diagnosis of T2DM by a physician, utilization of oral diabetes medications, HbA1c levels ≥ 6.5%, and fasting blood glucose levels ≥ 126 mg/dL (7 mmol/L). Participants were required to exhibit a sedentary lifestyle, characterized by the absence of regular exercise for six months preceding the study. This criterion was further evaluated through the completion of the International Physical Activity Questionnaire (IPAQ) for self-reported data. All participants had to provide written informed consent and agree to full cooperation throughout the study duration. Blood samples (10 mL) were obtained from the antecubital vein of subjects in a seated position 24 hours prior to the initiation of the protocol and 48 hours after the final session following a 12-hour overnight fast. Fasting glucose was quantified using a Pars Azmoon kit (Iran, Tehran, Azmoon Pars) via the glucose oxidase method (Hitachi®, model 704, 902 manufactured in Japan).
3.5. Sample and Sampling Method
The analysis of blood samples for biomarkers such as transforming growth factor beta (TGF-β) [Human TGF-β enzyme-linked immunosorbent assay (ELISA) kit, catalogue number: SL1736Hu], APOE4 (human APOE ELISA kit, catalogue number: SL2790Hu), sTREM2 (human sTREM2 ELISA kit, catalogue number: SL2717Hu), TYROBP (human TYROBP ELISA kit, catalog number: RK12100), and human osteopontin (SPP1) (human SPP1, OPN ELISA kit, catalog number: E1525Hu) was conducted utilizing the ELISA methodology. The assay employed a sandwich technique, wherein a 96-well plate pre-coated with an anti-human SPP1 antibody was utilized. Standards ranging from 0.3 ng/mL to 90 ng/mL were prepared by serial dilution from a stock solution (96 ng/mL) using the provided standard diluent. The kit components, including the microplate, standards, and reagents, were utilized according to the manufacturer's instructions.
3.6. Intervention
Prior to commencing the protocol, patients in the intervention group received information regarding the necessary steps and precautions to ensure safe and correct administration of the therapy. They were taught the proper way to plan their meals before, during, and after physical activity, and to identify warning signs of low blood sugar, such as feeling unsteady or being uncomfortable. Participants were allocated into two exercise groups based on findings by Sato et al., with one group exercising in the morning and the other in the evening (16, 23). The training was conducted indoors at a controlled temperature of 25 - 26°C for 12 weeks, with three sessions per week (totaling 36 sessions). The morning group trained three hours after sunrise (ZT3, approximately 9:00 am), while the evening group trained three hours after sunset (ZT15, approximately 9:00 p.m.). The 12-week TPMP program included a warm-up, 30 minutes of brisk walking at 60 - 70% of maximum heart rate reserve, and a cool-down. Warm-ups featured kinetic movements and dynamic stretching, while cool-downs consisted of static stretches (24).
The control group, unlike the intervention groups, did not participate in any structured exercise program during the 12-week study. Participants were explicitly instructed to refrain from initiating new exercise programs or increasing the intensity/frequency of existing physical activities beyond their typical daily routines, and to avoid any activity resembling the intervention's aerobic regimen.
3.7. Cognitive Assessment
Cognitive function was assessed using the Addenbrooke’s cognitive examination (ACE), a comprehensive tool for diagnosing and differentiating types of dementia, particularly AD and frontotemporal dementia (FTD). The revised ACE version has achieved high diagnostic accuracy, with sensitivity rates of 0.88 and 0.82. The test comprises five subtests, evaluating attention/orientation (18 marks), memory (26 marks), verbal fluency (14 marks), language (16 marks), and visuospatial ability (16 marks) (25). In 2009, the Persian version of the ACE test underwent standardization in accordance with Iranian culture. The Cronbach's alpha coefficients for all participants, the control group, the mild cognitive impairment (MCI) group, and the AD group were 0.84, 0.97, 0.88, and 0.93, respectively, demonstrating the high internal consistency of this assessment instrument (26).
3.8. Statistical Analysis
Statistical analyses were conducted utilizing Graph Pad Prism 9.0.0 and IBM SPSS Statistics v27. The normality of data distribution was assessed employing the Shapiro-Wilk and Kolmogorov-Smirnov tests, with a significance level established at 0.05. The homogeneity of variance across groups was evaluated using Levene's test. Controlling for the pre-test effect, MANCOVA and ANCOVA were used to compare groups at each training time point to calculate posttest score differences for variables. Post hoc comparisons between groups were performed using Bonferroni tests.
4. Results
First, the number of people in each group and the average age of each group were determined, with each group having 15 participants. The mean ages were as follows.
(1) Morning practice: Mean age = 58.50 ± 2.50
(2) Evening practice: Mean age = 57.86 ± 2.50
(3) Control: Mean age = 59.10 ± 2.55
The descriptive statistics, including the mean and standard deviation for demographic variables such as Body Mass Index (BMI), blood sugar, and fat percentage, were calculated for the three groups: Morning exercise, evening exercise, and control (Table 2).
| Variables | Pre-test | Post-test |
|---|---|---|
| BMI (kg/m2) (N = 15) | ||
| Morning exercise | 31.55 ± 3.69 | 30.32 ± 3.35 |
| Evening practice | 30.11 ± 4.88 | 29.54 ± 4.57 |
| Control | 29.21 ± 4.00 | 29.43 ± 3.93 |
| Blood sugar (mg/dL) (N = 15) | ||
| Morning exercise | 179.85 ± 15.58 | 143.85 ± 16.67 |
| Evening practice | 151.92 ± 19.15 | 127.14 ± 16.68 |
| Control | 151.90 ± 17.89 | 159.40 ± 16.84 |
| Fat percentage (N = 15) | ||
| Morning exercise | 25.68 ± 4.77 | 25.35 ± 4.40 |
| Evening practice | 28.03 ± 3.99 | 26.88 ± 3.86 |
| Control | 27.15 ± 2.71 | 27.21 ± 2.69 |
Abbreviation: BMI, Body Mass Index.
a Values are expressed as mean ± SD.
It is important to note that the BMI and body fat percentage were calculated using the following formulas:
(1) Body Mass Index = Weight (kg)/height2 (m2)
(2) Body fat percentage = (495/density) - 450
Descriptive findings of this study include statistical indicators such as the mean and standard deviation for the blood biomarkers TYROBP, SPP1, sTREM2, APOE, and TGF-β, as presented in Table 3. Furthermore, cognitive function, as measured by the Addenbrooke's cognitive examination and its subscales (attention and orientation, memory, fluency, language, and visuospatial-spatial ability), is detailed for the three groups — morning exercise, evening exercise, and control — in Table 4.
| Variables | Pre-test | Post-test |
|---|---|---|
| TYROBP (pg/mL) (N = 15) | ||
| Morning exercise | 28.51 ± 6.54 | 24.08 ± 5.05 |
| Evening practice | 31.32 ± 11.29 | 12.66 ± 8.39 |
| Control | 41.33 ± 9.32 | 30.05 ± 7.40 |
| SPP1 (ng/mL) (N = 15) | ||
| Morning exercise | 13.59 ± 4.25 | 7.25 ± 1.86 |
| Evening practice | 13.21 ± 4.43 | 9.52 ± 0.95 |
| Control | 10.97 ± 3.78 | 20.12 ± 2.26 |
| sTREM2 (pg/mL) (N = 15) | ||
| Morning exercise | 159.52 ± 31.50 | 114.58 ± 14.44 |
| Evening practice | 127.58 ± 40.80 | 93.87 ± 11.46 |
| Control | 128.52 ± 32.75 | 160.92 ± 33.2 |
| APOE (ug/mL) (N = 15) | ||
| Morning exercise | 24.55 ± 9.99 | 30.05 ± 5.95 |
| Evening practice | 28.03 ± 12.76 | 51.48 ± 6.83 |
| Control | 29.70 ± 12.83 | 33.46 ± 10.26 |
| TGF-β (ng/mL) (N = 15) | ||
| Morning exercise | 34.45 ± 15.13 | 20.44 ± 5.72 |
| Evening practice | 36.96 ± 12.25 | 26.01 ± 7.02 |
| Control | 38.18 ± 16.41 | 45.03 ± 13.63 |
Abbreviations: TYROBP, TYRO protein tyrosine kinase-binding protein; SSP1, Osteopontin; sTREM2, soluble triggering receptor expressed on myeloid cells 2; APOE, apolipoprotein E; TGF-β, transforming growth factor beta.
a Values are expressed as mean ± SD.
| Variables | Pre-test | Post-test |
|---|---|---|
| Attention and orientation (N = 15) | ||
| Morning exercise | 15.64 ± 1.17 | 16.43 ± 1.86 |
| Evening practice | 15.14 ± 1.17 | 16.79 ± 1.57 |
| Control | 15.10 ± 0.99 | 15.10 ± 0.99 |
| Memory (N = 15) | ||
| Morning exercise | 16.43 ± 1.03 | 19.86 ± 1.93 |
| Evening practice | 16.21 ± 1.47 | 20.29 ± 1.84 |
| Control | 16.00 ± 1.41 | 16.60 ± 0.84 |
| Fluency of speech (N = 15) | ||
| Morning exercise | 7.36 ± 1.69 | 8.79 ± 2.00 |
| Evening practice | 7.00 ± 1.61 | 8.64 ± 1.98 |
| Control | 7.20 ± 1.22 | 7.20 ± 1.22 |
| Language (N = 15) | ||
| Morning exercise | 18.71 ± 2.17 | 22.21 ± 2.63 |
| Evening practice | 20.14 ± 2.28 | 23.79 ± 2.39 |
| Control | 20.50 ± 1.95 | 21.10 ± 1.52 |
| Visual-spatial ability (N = 15) | ||
| Morning exercise | 11.93 ± 2.84 | 13.43 ± 2.20 |
| Evening practice | 12.50 ± 2.68 | 13.86 ± 2.47 |
| Control | 9.20 ± 2.30 | 9.80 ± 2.70 |
| Cognitive function (Addenbrook) (N = 15) | ||
| Morning exercise | 70.07 ± 6.73 | 80.71 ± 8.25 |
| Evening practice | 71.00 ± 6.34 | 83.35 ± 7.79 |
| Control | 68.00 ± 4.80 | 70.30 ± 4.13 |
a Values are expressed as mean ± SD.
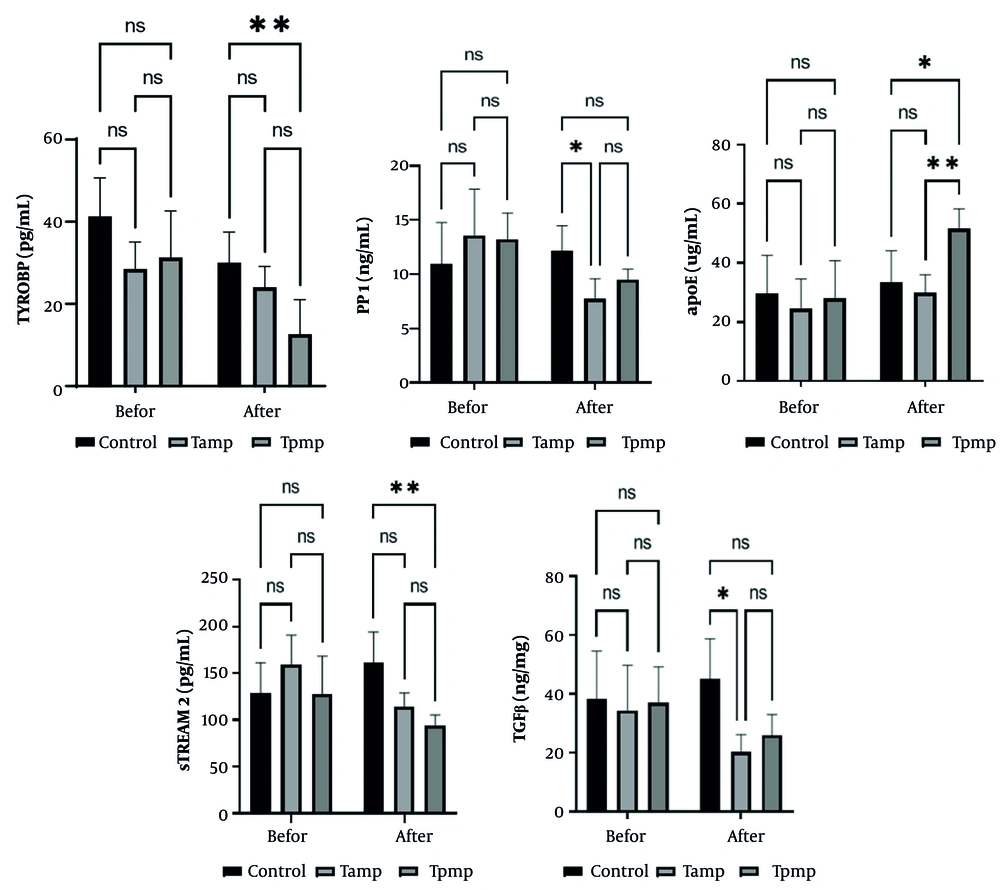
Statistical analysis revealed significant differences in several markers compared to the control group. The TYROBP and sTREM2 levels were significantly elevated in the afternoon aerobic exercise group (TPMP) (P < 0.01). The TGF-β and SPP1 levels were significantly elevated in the morning TPMP group (P < 0.05). The APOE protein levels were highest in the morning TPMP group, demonstrating a statistically significant increase compared to both the control group (P < 0.05) and the TAMP group (P < 0.01) (Figure 1).
The study comprised three distinct groups: (A) a diabetic control group (control), (B) a group engaging in TAMP, and (C) a group participating in afternoon TPMP. Statistical significance was determined with a threshold of P < 0.05. Differences between groups were indicated by asterisks: *, **, ***, and **** correspond to significance levels of P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively. The notation 'Ns' denotes a lack of statistical significance between groups.
To evaluate the impact of morning and evening aerobic activities on cognitive performance and Addenbrooke's cognitive examination subtests in the elderly, an analysis of covariance (MANCOVA & ANCOVA) was employed. Kolmogorov-Smirnov and Shapiro-Wilk tests were used to check the normality of the data, and Levene's test was used to ensure homogeneity of variances. The results of these tests indicated that the distribution of scores in the groups was normal (P > 0.05). Also, the results of the M-box test were examined to ensure that the assumption of the same variance-covariance matrix was not violated. The results showed that this assumption was not violated (1.16, F = 0.05, P = 0.19). The results are presented in Tables 5 and 6.
| Title of Exam | Amount | F-Value | Degrees of Freedom of the Hypothesis | Error Degrees of Freedom | P-Value | Partial Eta Squared | Observed Power |
|---|---|---|---|---|---|---|---|
| Pillai effect | 0.77 | 3.44 | 10 | 54 | 0.001 | 0.39 | 0.98 |
| Wilks lambda | 0.32 | 3.97 | 10 | 52 | 0.000 | 0.43 | 0.99 |
| Hoteling’s work | 1.80 | 4.51 | 10 | 50 | 0.000 | 0.47 | 0.99 |
| The largest root | 1.61 | 8.69 | 5 | 27 | 0.000 | 0.61 | 0.99 |
| Dependent Variables | Sum of Squares | df | Mean Square | F | P-Value | Partial Eta Squared | Observed Power |
|---|---|---|---|---|---|---|---|
| Attention and orientation | 10.76 | 0.00 | 0.41 | 0.98 | |||
| Contrast | 9.65 | 2 | 4.82 | ||||
| Error | 13.44 | 30 | 0.44 | ||||
| Memory | 11.98 | 0.00 | 0.44 | 0.99 | |||
| Contrast | 57.23 | 2 | 28.61 | ||||
| Error | 71.66 | 30 | 2.38 | ||||
| Fluency of speech | 5.96 | 0.00 | 0.28 | 0.84 | |||
| Contrast | 5.78 | 2 | 2.89 | ||||
| Error | 14.55 | 30 | 0.48 | ||||
| Language | 7.14 | 0.00 | 0.32 | 0.90 | |||
| Contrast | 27.27 | 2 | 13.63 | ||||
| Error | 57.26 | 30 | 1.90 | ||||
| Visual-spatial ability | 2.56 | 0.04 | 0.20 | 0.77 | |||
| Contrast | 7.24 | 2 | 3.62 | ||||
| Error | 42.46 | 30 | 1.41 | ||||
| Cognitive performance (Addenbrooke) | 20.57 | 0.00 | 0.57 | 1.00 | |||
| Contrast | 418.19 | 2 | 209.09 | ||||
| Error | 304.89 | 30 | 10.16 |
Next, the significance levels of the Wilks Lambda test showed that there was a significant difference in the cognitive function variable between the pre-test and post-test in the three experimental groups: (A) Morning exercise, (B) evening exercise, and (C) control group (Wilks Lambda: 0.22, 3.06, F = 0.003, P = 0.003). Therefore, exercise training has been able to affect the cognitive function variable, and the main hypothesis of the study — that exercise training has an effect on the cognitive function variable — is confirmed.
The results presented in Table 6 indicate that the F statistic for aerobic activities, after adjusting for all variables, is significant at the P < 0.00 level. This suggests that there is a statistically significant difference in cognitive performance and its subscales among the three groups. The power of the test for each variable further confirms that these differences are substantial within the population. These findings suggest that both morning and evening aerobic activities have positively influenced cognitive performance in the elderly. To identify which specific groups exhibit these effects, Bonferroni's post hoc test results are provided in Table 7.
| Variables | Difference of Means | Standard Deviation Error | P-Value |
|---|---|---|---|
| Attention and orientation | |||
| Evening practice | |||
| Morning exercise | 0.72 | 0.24 | 0.01 |
| Control | 1.65 | 0.27 | 0.00 |
| Morning exercise | |||
| Control | 0.92 | 0.27 | 0.00 |
| Memory | |||
| Evening practice | |||
| Morning exercise | 0.61 | 0.64 | 1.00 |
| Control | 3.50 | 0.70 | 0.00 |
| Morning exercise | |||
| Control | 2.88 | 0.70 | 0.00 |
| Fluency of speech | |||
| Evening practice | |||
| Morning exercise | 0.24 | 0.26 | 1.00 |
| Control | 1.16 | 0.28 | 0.00 |
| Morning exercise | |||
| Control | 0.91 | 0.28 | 0.01 |
| Language | |||
| Evening practice | |||
| Morning exercise | 0.64 | 0.62 | 0.92 |
| Control | 2.91 | 0.66 | 0.00 |
| Morning exercise | |||
| Control | 2.27 | 0.69 | 0.00 |
| Visual-spatial ability | |||
| Evening practice | |||
| Morning exercise | -0.04 | 0.42 | 1.00 |
| Control | 1.35 | 0.52 | 0.04 |
| Morning exercise | |||
| Control | 1.39 | 0.50 | 0.02 |
| Cognitive function (Addenbrooke's) | |||
| Evening practice | |||
| Morning exercise | 1.54 | 1.23 | 0.65 |
| Control | 10.29 | 1.40 | 0.00 |
| Morning exercise | |||
| Control | -8.75 | 1.40 | 0.00 |
Post-test scores for the experimental groups (morning exercise and evening exercise) and the control group were compared to pre-test scores. The Bonferroni test results presented in the table reveal significant differences (P < 0.001) across all cognitive performance variables among the three groups: (A) morning exercise, (B) evening exercise, (C) and the control group. This indicates that both intervention methods — morning and evening TPMP — significantly improved cognitive performance in the elderly compared to the control group. However, the effects of morning and evening aerobic activities differed, with evening exercise demonstrating a superior impact on cognitive performance relative to morning exercise, as reflected by the average scores in the two intervention groups.
5. Discussion
The T2DM is known to increase the risk of AD through several mechanisms, including increased inflammation (e.g., TGF-β), immune cell dysfunction in the brain (e.g., TYROBP and sTREM2) (27, 28), and altered signaling pathways (e.g., SPP1) (29). In addition, dysregulated immune responses contribute to the pathogenesis of AD. Research has shown that modulating microglial activity, improving Aβ clearance, or reducing neuroinflammation via the TYROBP/sTREM2 axis is critical for attenuating the cognitive decline associated with AD (30, 31).
This study aimed to investigate the efficacy of different exercise sessions — morning or evening — in modulating these factors in older women with T2DM. One of the main findings was that TYROBP and sTREM2 levels were significantly lower in the evening TPMP group compared to the control group. In addition, TGF-β and SPP1 levels were significantly reduced in the TAMP compared to the control group. The evening exercise group also showed a significant increase in APOE levels compared to the morning exercise and control groups. These results suggest that physical exercise may influence inflammation and neuroplasticity-related factors, which may have a protective effect against the cognitive decline associated with aging.
Previous studies have shown that long-term exercise programs can improve cognitive function, but short-term interventions appear to have limited effects (32). This study shows that several genes, including TYROBP, sTREM2, TGF-β, APOE4, and SPP1, are involved in the observed cognitive benefits, suggesting that physical activity may serve as a therapeutic strategy for AD.
Animal studies have shown that physical exercise has neuroprotective effects, including improving memory, learning ability, spatial memory, and reducing anxiety (33). Possible mechanisms by which physical activity may prevent neurodegenerative diseases such as Alzheimer's include:
Microglia, the brain's immune cells, are critical to maintaining brain health by clearing debris and regulating inflammation. Exercise can improve the function of microglia and thus their ability to remove harmful substances (34). Exercise can promote the survival and resilience of neurons, helping to maintain cognitive abilities (35).
The sTREM2, a protein mainly expressed in microglia, plays a crucial role in the regulation of inflammatory responses, lipid metabolism, and phagocytosis. Dysfunctional sTREM2 can impair these processes, hindering the clearance of Aβ plaques and contributing to neurodegeneration. sTREM2 is also involved in the amyloid and tau pathologies associated with AD (36).
The TYROBP, a downstream adapter protein for sTREM2, influences its signaling pathway. Recent research has uncovered interactions between the APOE ε4 allele and the TYROBP/sTREM2 signaling pathway that significantly affect microglial functions, including Aβ phagocytosis. This can lead to neuroinflammation and neuronal apoptosis, contributing to the development of AD (28, 37).
Individuals carrying the APOE ε4 allele have lower levels of APOE protein, which interferes with cholesterol transport and potentially exacerbates AD pathology. In addition, the lack of APOE protein in these individuals impedes microglial suppression and the reduction of proinflammatory cytokines, contributing to neuroinflammation and increased Aβ pathology (38).
The reduced sTREM2 levels we found in the exercise groups are consistent with previous research showing that exercise can regulate sTREM2 function, possibly leading to cognitive benefits in AD patients (31). Another study showed that exercise reduces the activation of pro-inflammatory microglia and lowers plasma levels of sTREM2, indicating reduced systemic inflammation (4).
A study suggested that exercise may prevent sTREM2 degradation and thus preserve hippocampal glucose metabolism and cognitive function in AD models (30). In addition, a five-year study examined the interaction between the APOE genotype and physical activity and showed that regular physical activity reduces the risk of dementia, especially in individuals without the APOE ε4 allele (39).
The TGF-β is another interesting protein that plays an important role in the development of insulin resistance, obesity, and DM (40). Elevated TGF-β levels contribute to AD through its involvement in the formation of Aβ plaques (41). While exercise increases the production of ROS, which can be harmful in high concentrations, it also boosts the body's antioxidant defenses, which counteract ROS and lower TGF-β levels over time (42).
The SPP1, another protein studied, plays a crucial role in the inflammatory cascade and can impair insulin signaling, which contributes to neurological diseases such as AD (23). Our study observed a decrease in SPP1 protein levels in the exercise groups, particularly in the morning exercise group, which is consistent with previous research showing that long-term running improves cognitive function through SPP1 modulation (4).
Circadian biology, particularly the rhythms of cortisol and melatonin, plays a crucial role in timing-dependent physiological effects. These hormones are integral to the body's internal clock, influencing various biological processes and health outcomes. Cortisol is a key hormone in maintaining circadian rhythm, with its release peaking in the early morning to prepare the body for the day (43). The SPP1 induces the activation of TGF-β release signaling. Furthermore, several studies have demonstrated a strong correlation between TGF-β 1 and cortisol levels. Given that cortisol secretion is typically higher in the morning compared to the evening, the regulation or reduction of cortisol secretion through exercise may effectively contribute to the decrease in TGF-β and SPP1 levels (2).
Melatonin is synthesized at night and is pivotal in distributing temporal cues from the SCN to peripheral organs. Decreased melatonin levels are associated with T2DM, which in turn triggers neuroinflammation (44). Exercise has been shown to increase melatonin secretion (45); therefore, the observed decrease in TYROBP and sTREM2 levels during evening workouts can be attributed to this melatonin elevation.
Furthermore, our results suggest that both morning and evening exercise significantly improve cognitive performance in older people. This is consistent with previous studies that have shown that regular exercise can improve cognitive function in older adults (15, 34, 46). For example, one study showed that low-intensity exercises such as balance and coordination exercises can help prevent age-related cognitive decline in the early stages of AD (47). These improvements are likely due to increased performance of the prefrontal cortex, the brain region responsible for executive functions. In addition, physical activity is known to increase neurotrophic factors and neurotransmitters, improve neurogenesis and plasticity in the brain, and enlarge neuronal structures such as the dentate gyrus (48).
5.1. Conclusions
In conclusion, while recognizing the critical importance of individual circumstances, preferences, and lifestyle factors in personalized exercise prescription, this study highlights the significant influence of exercise timing on the modulation of specific physiological pathways. The 12-week TPMP program demonstrated beneficial effects on certain health markers in women with T2DM. However, it is important to note that although exercise timing did not result in significant changes across all measured variables, it did produce time-dependent effects on specific biomarkers. A more comprehensive understanding of these time-dependent effects will aid in the development of more tailored and effective exercise interventions. This suggests that personalized exercise recommendations, incorporating the time of day, could serve as a targeted intervention strategy to reduce AD risk in this population. Ultimately, by refining the understanding of the mechanistic links between exercise timing and specific biomarkers, more precise and effective exercise strategies for at-risk populations can be developed.
5.2. Limitations
While this study suggests positive impacts of exercise, it's crucial to acknowledge several inherent limitations. Firstly, the participant pool, restricted to elderly individuals from Shahrekord, may limit the generalizability of our findings to broader, more diverse populations. Secondly, the small sample size (n = 15/group) risks underpowered results. Moreover, we recognize that potential confounding variables, notably dietary intake and sleep architecture, were not subject to rigorous control. Future research should implement standardized protocols for these factors to isolate the independent effects of the primary variables under investigation. Additionally, integrating qualitative methodologies alongside quantitative tools could provide richer, more nuanced insights. The short intervention duration (12 weeks) and the absence of long-term follow-up also pose limitations. Consequently, the interpretation of our present findings must be carefully contextualized, acknowledging the potential for bias introduced by these uncontrolled variables, which may have influenced the observed outcomes. Promoting awareness among elderly individuals and their families through mass media, educational resources, and training sessions is essential to disseminate the benefits of physical activity.