1. Background
Epilepsy is a common disease of CNS with signs of seizures (1), lack of consciousness and abnormal activity of autonomous system. The incidences of epilepsy in the world is 0.5 to 2% and it may occur at any age (1). In addition, it is usually accompanied with psychological, emotional and learning disturbances. Typically, preventive and therapeutic approaches are with some undesired adverse effects (1), but usually herbal therapeutics show no adverse effects.
E. caucasicum belongs to the family of Apiaceae (Umbelliferae) (2). The literature on the possible activities of Eryngium species only highlights the anti-inflammatory and antinociceptive properties (2); however, numerous medicinal uses were reported for this plant (stimulant, diaphoretic, diuretic, stone inhibitor, aphrodisiac, expectorant and anthelmintic) (2). Moreover, antioxidant activities of the leaf of this plant have just been reported recently (2, 3), showing a very good antioxidant activity (4); it’s antihypoxic and renoprotective effects have also been reported recently (4, 5).
2. Objectives
Because of its good antioxidant, antihypoxic and CNS activities, this plant was selected for the assay. In this study, we examined the anticonvulsant activities of methanolic extracts of E. caucasicum on maximal electroshock (MES) and pentylenetetrazole (PTZ)-induced seizures in mice.
3. Materials and Methods
3.1. Chemicals
Pentylenetetrazole was purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Diazepam was purchased as 10 mg/ml ampoule from Tolid-daru Co. Tehran, Iran.
3.2. Experimental Animals
The protocol for the study was based on animal ethical committee of Mazandaran University of Medical Sciences. Swiss male albino mice (20 - 25 g, Pasteur institute of Iran) were used for this study. The animals were housed in standard cages with free access to food and water. The temperature was maintained at 23 ± 1°C with a 12-hour light/12-hour dark cycle. Each animal was used once. Inclusion criteria for animals were their sex (male) and race (Swiss albino) and habitation in the lab environment one week before the exam. The exclusion criteria were being used previously for any behavioral exams and having underlying diseases.
3.3. Plant Material and Preparation of Freeze-Dried Extract
E. caucasicum inflorescence was collected from Sari, Iran, in June 2013. The sample was authenticated by Dr. Bahman Eslami, and the voucher specimen was deposited (No: 1725) in the herbarium of the Biology School of Gaemshahr Azad University. Plant materials were dried under dark conditions at the room temperature. The dry material was coarsely grounded (2 - 3 mm) and then extracted by methanol for 24 hours at the room temperature. The extracts were then separated from the sample residues by filtration. Extraction was repeated thrice. The resulting extracts were concentrated over a rotary vacuum at 35 - 40°C until crude solid extracts were obtained, which then were freeze-dried for complete solvent removal.
3.4. Anticonvulsant Activity
3.4.1. Convulsions Induced by Pentylenetetrazol
Seizure induced by PTZ was performed to evaluate the anticonvulsant property of the extract. Seventy-seven male mice were divided into eight groups, each of which comprising nine mice. The groups were treated with normal saline (10 ml kg-1), diazepam (4 mg kg-1), and methanolic extracts of E. caucasicum at doses of 125, 250, and 500 mg kg-1, and poly phenolic extracts at doses of 50,100, 200 mg kg-1 separately. Thirty minutes later, convulsions were induced by the intraperitoneally administration of 100 mg kg-1 of PTZ. Following the administration of PTZ, the mice were placed in separate transparent Plexiglas cages (25 × 15 × 10 cm) and were observed for the occurrence of seizures over a 30-minute period. The latency of the first convulsive episode, duration of tonic convulsions, and percentage of deaths were recorded (6).
3.5. Maximal Electroshock (MES) Induced Convulsions
Forty-eight male mice were divided into eight groups, each of which having six mice. The groups were treated with normal saline (10 mm kg-1), diazepam (4 mg kg-1), and methanolic extracts of E. caucasicum at doses of 125, 250, and 500 mg kg-1, and polyphenolic extracts at doses of 50, 100, 200 mg kg-1 separately. After 30 minutes, convulsions were induced in all the groups of animals, using electro-convulsometer. A 50 Hz alternating current of 60 mA for 0.2 seconds was delivered through the ear electrodes (7). The incidence and duration of tonic hind limb extension was recorded.
3.6. Statistical Analysis
Data as mean ± SD were analyzed by ANOVA. Duncan’s new multiple range test was used to determine the differences in means. All P values less than 0.05 were considered significant.
4. Results
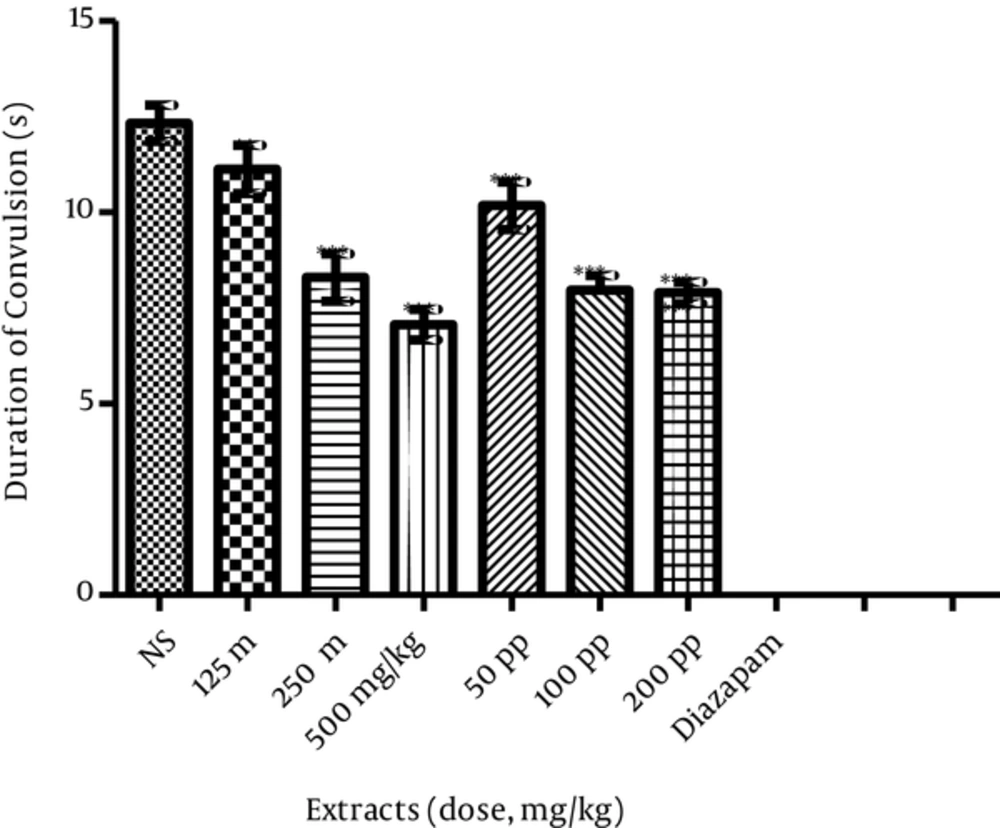
Maximal electroshock produced hind limb tonic extension in all the control animals. Normal saline treated mice showed tonic hind limb extension for 12.33 ± 0.48 seconds. The onset was 2.75 ± 0.68 seconds. Methanolic and polyphenolic extracts increased the onset of seizures induced by MES and significantly decreased the duration of tonic hind limb extension (Table 1). Methanolic extract at 250 and 500 mg kg-1 and polyphenolic extract at 200 mg kg-1 induced maximum protection against seizures, and polyphenolic extract at 200 mg kg-1 had 66.6% protection. Methanolic extract at 250 and 500 mg kg-1 induced 50% protection against seizures. The minimum length of the seizure (500 mg kg-1) was 7.07 ± 0.45 seconds after methanolic extract. The maximum length of seizure after methanolic extract (125 mg kg-1) was 11.13 ± 0.63 seconds. No convulsion was observed, and 100% protection was found in mice treated by Diazepam at 4 mg kg-1 (Figure 1).
| Dose PTZ | Dose Extract | Dose Diazepam | No. of Animals Convulsed/Used | Mortality Protection, (%) | Onset of Tonic Convulsion (Mean ± SD) (Second) |
|---|---|---|---|---|---|
| 100 | 125 M | - | 9/9 | 11 | 37.00 ± 13.06b |
| 100 | 250 M | - | 9/9 | 22 | 68.29 ± 16.70c |
| 100 | 500 M | - | 9/9 | 22 | 81.43 ± 12.49c |
| 100 | 50 P | - | 9/9 | 11 | 23.56 ± 9.59b |
| 100 | 100 P | - | 9/9 | 22 | 59.13 ± 19.98c |
| 100 | 200 P | - | 8/9 | 22 | 103.00 ± 6.36c |
| 100 | - | - | 9/9 | 0 | 16.56 ± 4.7 |
| 100 | - | 2 | 0/9 | 100 | 129.7 ± 14.17c |
| - | 125 M | - | 4/6 | 33.3 | 3.77 ± 0.46b |
| - | 250 M | - | 3/6 | 50 | 5.70 ±0.62c |
| - | 500 M | - | 3/6 | 50 | 7.07 ± 0.45c |
| - | 50 P | - | 4/6 | 33.3 | 3.33 ±0.31b |
| - | 100 P | - | 4/6 | 33.3 | 6.98 ± 0.17c |
| - | 200 P | - | 2/6 | 66.6 | 9.15 ± 0.21c |
| - | - | - | 6/6 | 0 | 2.75 ± 0.681 |
| - | - | 2 | 0/6 | 100 | Not convulsed |
aDoses are in mg/kg. Each group represents the mean ± SD.
bP < 0.01.
cP < 0.001 vs. control.
PTZ (100 mg kg-1) induced tonic seizures in 100% of the control mice. Pretreatment with methanolic and polyphenolic extracts increased significantly, and the onset of seizures was induced by PTZ (Table 1). The most rapid onset of seizure relating to polyphenolic extract (50 mg kg-1) was 23.56 ± 9.59 seconds, and it was 103.0 ± 6.36 seconds at 200 mg kg-1. The lowest percentage of deaths for polyphenolic extract was at 100 and 200 mg kg-1 and it was at 250 and 500 mg kg for methanolic extract-1. Diazepam showed a significant anticonvulsant activity at 4 mg kg-1, causing a 100% protection in mortality of mice.
5. Discussion
Kindling and MES (Maximal electroshock) are common models used in anticonvulsant studies of new drugs in animals, and for kindling some chemicals as pentylentetrazole (PTZ), bicoculine or picrotoxine were used (6-8). Recently, herbal medicine has been used for many pathological conditions such as diabetes, depression, seizures and other diseases. It was found that several plants possess neurobehavioral activities, and therefore, they have been used as alternatives in modern medicine (7). In this study, we aimed to assess the anticonvulsant effects of methanolic extracts of flowering shoots of E. caucasicum. MES and PTZ, as the current models of seizure study, were used for anticonvulsant assays. The results revealed that these extracts had anticonvulsant activities. The effect of most of the antiepileptic drugs are due to their inhibitory synaptic potential (such as chloride channels agonists) or to their preventive excitatory synaptic potential (Na+ or Ca++ Channel blockers). Moreover, recently some new approaches have been revealed to show the role of some herbal extracts with anticonvulsant activities (8). Those herbs with polyphenol or flavanoids constituents are good candidates for anticonvulsant studies because of their high scavenging properties for free radicals in the brain and for their ability to diminish glutamtergic toxicities. According to this study, the E. caucasicum extract prevented the PTZ convulsions. Moreover, as one of the PTZ's mechanisms for seizure is the antagonistic property against GABA receptors, it may be considered for GABA ergic agonistic effect for the anticonvulsant activity of the E. caucasicum extract. However, to determine the precise mechanism for this herbal extract, conducting complementary studies are necessary. MES-induced tonic seizures and seizures of PTZ can be prevented either by drugs that inhibit voltage-dependent Na+ channels or by drugs that block glutaminergic excitation mediated by the N-methyl-D-aspartate receptor (9). Antiepileptic drugs such as sodium valproate, which is effective in both types of seizures, possess multiple mechanisms of action and display the broadest therapeutic utility.
In different studies about Eryngium species, it was demonstrated that the polyphenol and flavanoids components with antioxidant and free-radical scavenger and antioxidative stress properties could explain its effectiveness against some diseases as neurodegenerative, cardiovascular and cancer (5, 10, 11). Therefore, the anticonvulsant efficacy of Eryngium might belong to its flavanoids quantities, but some other complementary exams are necessary to make clearer its anti-seizure mechanism. Considering the results of this study, it is recommended further research be conducted on the anticonvulsant effect of this herbal extract to precisely determine its main constituents with anticonvulsant activities. Moreover, we had some financial and instrumental limitations to distinguish the kind of flavonids of this extract for its pharmacologic properties.
5.1. Conclusion
According to this study, the E. caucasicum extract prevented the PTZ kindling and MES models of convulsions in mice, which were comparable with Diazepam. GABA ergic potentiating or radical scavengeric mechanism could be suggested for this pharmacologic activities. Furthermore, the polyphenol and flavanoids constituents of this herbal extract might be responsible for its anticonvulsant properties, but more studies are needed to clarify the pharmacologic properties of this herbal medicine.