1. Context
In a classic 1970 publication, the famous epidemiologist Alvan R. Feinstein defined comorbidity regarding a specific index condition, as any distinct additional entity that exists or may occur during the clinical course of a patient with the index disease under study (1). In the Feinstein formula, the implication was a different and independent disease occurred at the same time as another disease.
On the contrary, the diagnostic and statistical manual of mental disorders (DSM) explicitly produces overlapping clinical criteria for many diagnoses, especially mood and anxiety disorders, guaranteeing comorbidity in a quite different sense than in the medical meaning of the term as co-occurrence of independent diseases.
Psychiatric comorbidity is extremely common in bipolar disorder (BD). More than half of patients with BD have an additional disorders (2), one of the most difficult to manage is obsessive-compulsive disorder (OCD) (3-5).
However, although some authors recently investigated the co-occurrence of anxiety and BD, no meta-analysis regarding the prevalence or sub-group analyses specific to BD-I and BD-II were performed (6).
The present paper is the first systematic review and meta-analysis on the prevalence and predictors of comorbid BD-I/BD-II and OCD.
2. Objectives
The current review upgraded a previous systematic review and performed a meta-analysis to define the prevalence and predictors of comorbid BD-I/BD-II and OCD.
3. Data Sources
As done before (7), the review was conducted according to the methods recommended by the Cochrane collaboration (8) and the meta-analysis of observational studies in epidemiology (MOOSE) guidelines (9), and documented the process and results in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (10).
3.1. Information Sources and Search Strategy
Studies were identified searching the electronic databases MEDLINE, Embase, PsycINFO and the Cochrane Library. Authors combined the search strategy of free text terms and exploded MESH headings for the topics of bipolar disorder and obsessive-compulsive disorder combined as follows: bipolar disorderor (BD), bipolar or manic depressive disorder, manic depressive, manic and obsessive-compulsive disorder (OCD), obsessive-compulsive. The strategy was first developed in MEDLINE and then adaptedto the other databases (appendix 1. in supplementary file). Studies published in English untill June 30, 2015 were included. In addition, further studies were retrieved from reference listing of relevant articles and consultation with experts in the field.
3.2. Inclusion Criteria
3.2.1. Study Population and Study Design
The studies that included subjects with BD and OCD with specified diagnostic criteria were considered. Studies that considered subjects with bipolar and obsessive-compulsive spectrums with specified diagnostic criteria were also included (11, 12). Participants of both genders older than six years were considered. Studies conducted on subjects with physical comorbidities were excluded as non-representative of the study population (13, 14).
Both population- and hospital-based studies were included. Among hospital-based studies, inpatients, day-hospital and outpatient subjects were included while emergency care records were excluded as non-representative. All experimental and observational study designs were included apart from case reports. Narrative and systematic reviews, letters to the editor and book chapters were excluded.
3.2.2. Outcome Measures
Primary outcomes were i) lifetime prevalence of comorbid OCD in patients with BD-I/BD-II and ii) lifetime prevalence of comorbid BD-I/BD-II in patients with OCD. Studies that reported data only about the current prevalence were excluded (15). Secondary outcomes were potential predictors of comorbidity between BD-I/BD-II and OCD.
4. Study Selection and Data Extraction
Identified studies were independently reviewed for eligibility by two authors in a two-step process; a first screening was performed based on title and abstract while full texts were retrieved for the second screening. At both stages disagreements by reviewers were resolved by consensus. Data were extracted by one author and supervised by a second one using an ad-hoc developed data extraction spreadsheet. The data extraction spreadsheet was piloted on 10 randomly selected papers and modified accordingly.
4.1. Quality Assessment
The same authors who performed data extraction independently assessed the quality of selected studies using the checklist developed by Downs and Black, both for randomised and non-randomised studies (16). Disagreements by reviewers were resolved by consensus. Table 1 shows the quality assessment total score assigned to each study.
| References | Study Design | Country | Study Population | Sample Size | Diagnosis Assessment | Quality a |
|---|---|---|---|---|---|---|
| Population-Based Studies | ||||||
| Angst, J. et al. 2004 (11) | Prospective cohort study | Switzerland | 591 subjects recruited at age 19/20 and assessed over 20 years: OCD (n = 30) | 30 | Broad definition for BD and OCD; DSM-IV | 26/31 |
| Hospital-Based Studies: Adults | ||||||
| Bogetto, F. et al. 1999 (16) | Case-control study | Italy | OCD (n = 160, mean age: males 32.1 ± 13.0, females 36.9 ± 11.4 years) | 160 | NS; DSM-IV | 21/31 |
| Dilsaver, S.C. et al. 2008 (17) | Case-control study | USA | 187 Latino pt. enrolled consecutively from 2001 to 2003: BD-I (n = 69, mean age = 34.9 ± 11.8 years) | 69 | SCID-CV; DSM-IV | 20/31 |
| Hantouche, E.G. et al. 2003 (18) | Case-control study | France | OCD (n = 628, mean age CYC-OCD = 35 ± 12, mean age NC-OCD = 36 ± 14 years) | 628 | NS; DSM-IV | 24/31 |
| Kim, S.W. et al. 2014 (19) | Cross-sectional study | South Korea | BD-I (n = 174, age > 18 years) | 174 | SCID; DSM-IV | 22/31 |
| Lensi, P. et al. 1996 (20) | Case-control study | Italy | OCD (n = 263, mean age = 33.1 years) | 263 | NS; DSM-III-R | 21/31 |
| Maina, G. et al. 2007 (21) | Case-control study | Italy | OCD (n = 204, mean age = 34.7 ± 12.1 years) | 204 | SCID; DSM-IV | 21/31 |
| Marazziti, D. et al. 2002 (22) | Cross-sectional study | Italy | OCD (n = 117, mean age=30 ± 9.3 years) | 117 | SCID-P; DSM-IV | 21/31 |
| Perugi, G. et al. 1997 (23) | Case-control study | Italy | OCD (n = 315, mean age: BD-OCD = 32.8 ± 12.2, OCD = 32.5 ± 12.6 years) | 345 | NS; DSM-III-R | 22/31 |
| Perugi, G. et al. 1998 (24) | Case-control study | Italy | OCD (n = 135, mean age = 38.4 ± 13.3 years) | 135 | NS; DSM-III-R | 21/31 |
| Perugi, G. et al. 1999 (25) | Case-control study | Italy | 269 pt. enrolled consecutively from 1993 to 1995: OCD (n = 79, mean age = 30.4 ± 11.8 years) | 79 | SCID-Up-R; DSM-III-R | 20/31 |
| Perugi, G. et al. 2002 (26) | Case-control study | Italy | OCD-MDE (n = 68, mean age = 34.2 ± 12.5 years); BD-OCD (n = 38, mean age=35.9 ± 12.2 years) | 68 | SCID; DSM-IV | 20/31 |
| Shashidhara, M. et al. 2015 (27) | Cross-sectional study | India | BD-I (n = 396, age > 18 years) | 396 | SCID; DSM-IV | 23/31 |
| Timpano, K.R. et al. 2012 (28) | Case-control study | USA | OCD (n = 605, mean age = 39.2 years) | 605 | SCID-P; DSM-IV | 20/31 |
Studies Met the Inclusion Criteria for Systematic Review
4.2. Meta-Analysis
Individual study data were pooled using DerSimonian-Laird method (30) with comprehensive Meta-Analysis software ver. 3. Due to the anticipated heterogeneity, a random effects meta-analysis was employed. First, the data on the prevalence of BD-I and BD-II in patients with OCD were pooled separately; next the data on the prevalence of OCD in patients with BD-I and BD-II were pooled separately. Heterogeneity was assessed with the I2 and Q statistic for each analysis. Further, meta-regression analyses were conducted to investigate potential moderators if available data included (mean age, rate of males and duration of illness) with comprehensive Meta-Analysis ver. 3. Publication bias was assessed with a visual inspection of funnel plots (8), the Begg-Mazumdar Kendall's tau (31) and the Egger test (32).
5. Results
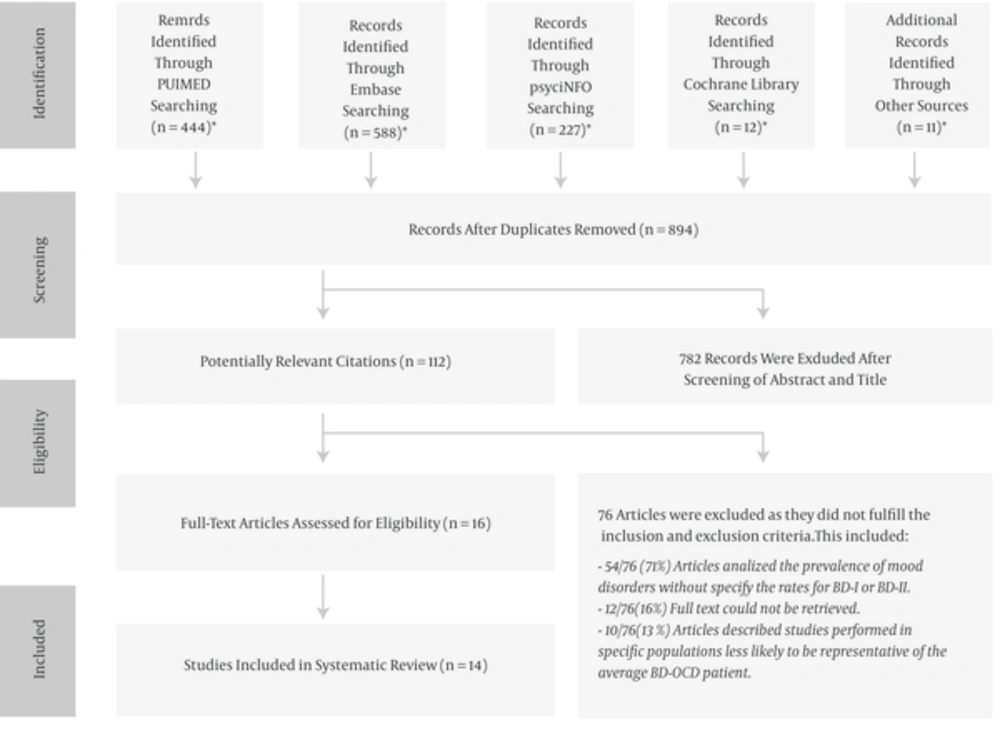
One thousand and two hundred eighty-two potential studies were identified by searching the selected databases and listing references of relevant articles. After removing duplicates, 894 articles were retrieved. Studies were screened and selected on the basis of pre-specified inclusion and exclusion criteria (Figure 1). The search identified 14 articles appropriate to be included in the systematic review: three articles about comorbid OCD in BD-I (8, 22, 32) and eleven articles about comorbid BD-I/BD-II in OCD (12, 17, 19, 21-27, 29).
5.1. Comorbid BD in OCD
5.1.1. Study Characteristics
The characteristics of included studies are reported in Table 1. Nine of the eleven studies were case-control studies, one cross-sectional study and one prospective cohort study. One study (9%) was population-based while the majority (n = 10, 91%) were hospital-based. Totally, 2,634 patients with OCD were represented among the eleven included studies. The mean age of patients with OCD was 34.7 ± 2.96 years and 43.8% were male (36.6%-50%). The majority of the studies were conducted in Europe (n = 9, 82%). In all the considered studies, diagnosis of BD-I/BD-II and OCD were based on the DSM criteria and they were established using validated assessment scales.
5.2. Meta-analysis of the pooled prevalence of BD-I/BD-II in patients with OCD
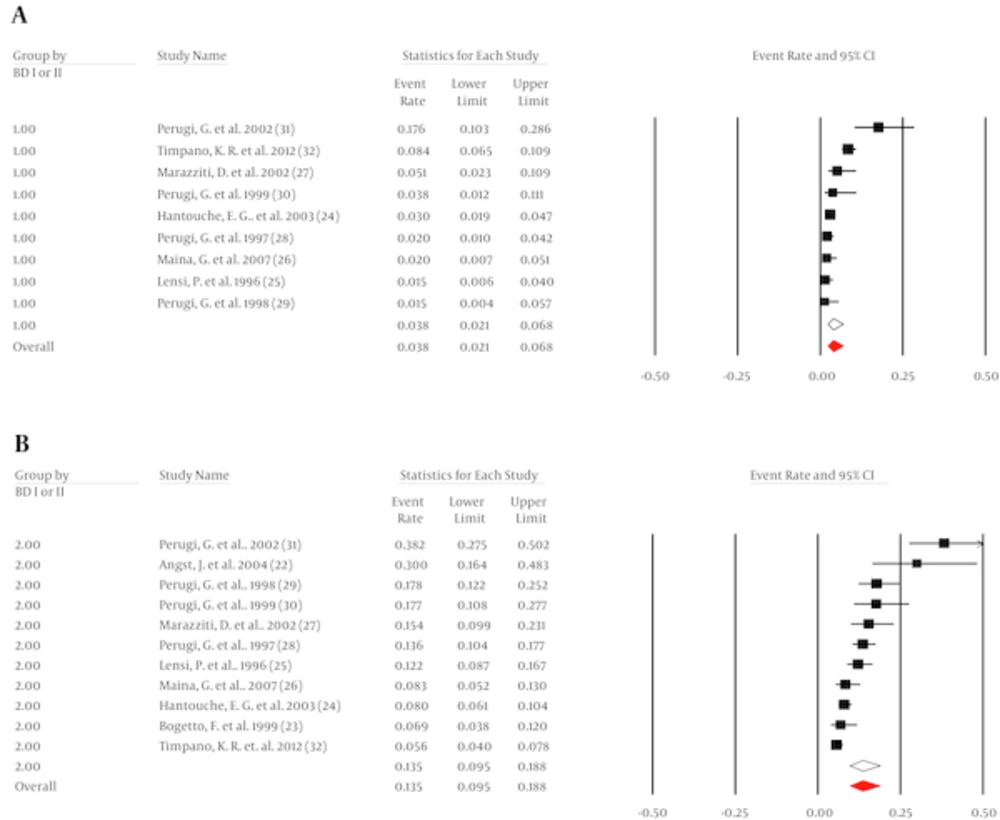
It was possible to pool data from 2,444 patients with OCD across 10 studies to found the prevalence of BD-I as 3.9% (95% CI, 2.4 to 6.4, I2 = 83%, Q = 56) (Figure 2A). Begg (tau = 1, P = 0.4) or Egger (intercept = -2.9, P = 0.19) did not indicate any evidence of publication bias.
It was possible to pool data from 2,634 patients with OCD across 12 studies to found the prevalence of BD-II as 13.5% (95% CI, 9.3 to 19.3, I2 = 89, Q = 91) (Figure 2B). There was no evidence of publication bias (Begg = 1.7, P = 0.2; Egger = 4.8, P = 0.15).
5.3. Predictors of the Prevalence of BD-I/BD-II in Patients with OCD
Mean age did not predict the prevalence of BD-I in OCD (β = 0.0731, 95% CI, -0.1097 to 0.256, z = 0.78), but it did explain some of the observed heterogeneity (R2 = 0.13). The rate of males did not moderate the prevalence of BD-I (β = 0.035, 95% CI, 0.2356 to 0.1656, z = -0.34). Mean age did not moderate the prevalence of BD-II (β = -0.0577, 95% CI, -0.1942 to 0.0788, z = -0.83), but it did explain a small amount of the observed heterogeneity (R2 = 0.08). Moreover, the rate of males was not related to the prevalence of BD-II (β = -0.0317, 95% CI, -0.1483 to 0.085, z =-0.53).
5.4. Comorbid OCD in BD
5.4.1. Study Characteristics
The characteristics of the included studies are reported in Table 1. Two studies were cross-sectional and one case-control. All the studies were hospital-based and conducted in non-European countries. Totally, 639 patients with BD-I were evaluated in the three included studies. The mean age of patients with BD was 36.8 ± 5.8 years and 45% were male (36% - 60.5%). In all the considered studies, diagnosis of BD-I and OCD were based on the DSM criteria and were established using validated assessment scales.
5.5. Meta-analysis of the pooled prevalence of OCD in patients with BD-I
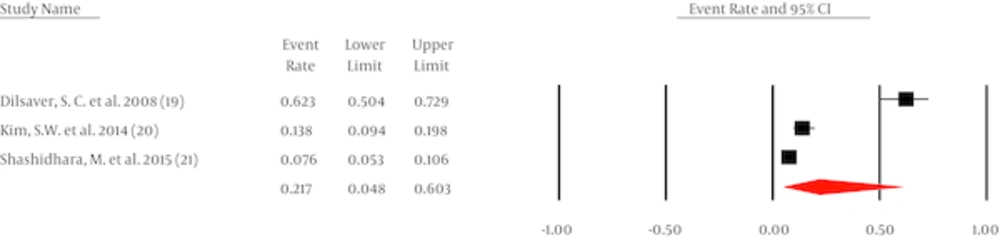
It was possible to pool data from three studies with 639 patients with BD-I, establishing a pooled prevalence of OCD at 21.7% (95% CI, 4.8 to 60.3, Q = 95, I2 = 84%) (Figure 3). Egger (intercept = 48, P = 0.2) and Begg (tau = 0.66, P = 0.29) did not indicate any publication bias.
6. Discussion
The entire question of comorbidity deserves attention, provided separately (33-36). In a standard 1969 psychiatry textbook, Mayer-Gross et al., mostly considering the course of illness, included patients with BD-OCD in the manic-depressive disorders (37). Although recent studies investigated the co-occurrence of anxiety and bipolar disorders, the topic is insufficiently studied and the relationship between BD and OCD remains unclear. However, given the available scientific evidence, some observations can be made.
Apparent BD-OCD comorbidity is a common condition in psychiatry. In the authors’ recent meta-analysis, the pooled prevalence of OCD in BD was 17.0% (95% CI, 12.7 to 22.4), which was comparable to the results reported by the pooled prevalence of BD in OCD (18.35%, 95% CI, 13.2 to 24.8) (33). Although limited by the retrospective study design, small sample size, different thresholds for BD diagnosis and a different accuracy in diagnosing OCD, these results suggest the relevance of comorbid BD-OCD.
The current meta-analysis found that the pooled prevalence of BD-I in OCD was 3.9% (95% CI, 2.4 to 6.4, I2 = 83%, Q = 56); while the pooled prevalence of BD-II in OCD was 13.5% (95% CI, 9.3 to 19.3, I2 = 89%, Q = 91). The pooled prevalence of OCD in BD-I was 21.7% (95% CI, 4.8 to 60.3, I2 = 84%, Q = 95). With regard to OCD-BD predictors, mean age and rate of males did not predict the prevalence of BD-I (β = 0.0731, 95% CI, -0.1097 to 0.256, z = 0.78; β = 0.035, 95% CI, -0.2356 to 0.1656, z = -0.34) and BD-II (β = -0.0577, 95% CI, -0.1942 to 0.0788, z = -0.83; β = -0.0317, 95% CI, -0.1483 to 0.085, z =-0.53) in OCD. Mean age explained some of the observed heterogeneity (R2 = 0.13; R2 = 0.08).
From the nosological perspective, considering the course of illness as a key diagnostic validator, especially among patients with a primary diagnosis of BD, the majority of cases with comorbid OCD appeared to be related to mood episodes (34). OC symptoms in patients with comorbid OCD appeared more often - and sometimes exclusively - during depressive episodes, and comorbid BD and OCD cycled together, with OC symptoms often remitting during manic/hypomanic episodes. On the contrary, especially among patients with a primary diagnosis of OCD, there was a substantial minority of comorbid BD-OCD that may represent true OCD separate from BD with OC symptoms that improve or worsen during mood episodes without being related to them.
Results of the authors recent meta-analysis showed higher comorbidity rates in youths (24.2%, 95% CI, 10.36 to 41.60, n = 345, z =-9.5) compared to adults (13.56%, 95% CI, 10.4 to 16.25, n = 4,539) (33). In other words, OC symptoms would initially coexist with BD symptoms and they would gradually tend to decrease in the adulthood (38, 39).
From a neurobiological perspective, BD mostly showed hypoactivity in orbitofrontal cortex (OFC) (i e, decision making, impulse control) and in dorsolateral prefrontal cortex (DLPFC) (i e, planning, attentional set shifting) with grey matter volume reduction associated to manic episodes (40), while OCD mainly presented hyperactivity of OFC with deficit in emotional processing (41). The overlap of similar cortical-subcortical circuits may partially explain the clinical features of patients with comorbid BD-OCD during the course of illness.
The clinical features of patients with comorbid BD-OCD would explain why OCD and BD symptoms respond to adequate mood stabilizer treatment (4, 6). Only in a minority of patients with persistent comorbid OCD, despite improvement in mood episodes, addition of low doses of antidepressants could be considered while strictly monitoring emerging symptoms of mania or mixed states (4). Benefit with neuroleptics was also observed, although a few reports of exacerbation of OC symptoms with neuroleptic agents existed (4). Deep brain stimulation also seems to carry risks of manic worsening, and thus may not be a useful intervention in BD-OCD (4).
Further original studies are needed to clarify BD-OCD comorbidity. In particular, considering the growing interest over the last decades in shared pathophysiologies across psychiatric disorders (42), studies addressing neurobiological substrates are essential to illuminate pathogenetic mechanisms that underlie comorbid BD-OCD.
6.1. Limitations
The main limitation of this systematic review is linked to the study design and analysis strategy of the included studies, as documented in the quality assessment scale used. Most studies were observational and based on retrospective assessments. The use of retrospective assessment scales with low sensitivity in discriminating true ego-dystonic obsessions from depressive ruminations may have biased results towards an overestimation of obsessive symptom prevalence. Some of them did not include a control group. Small sample size and enrollment of subjects mainly from BD-OCD outpatient units may limit generalizability of these results. Potential confounding factors in these studies include demographic and historical illness variables, which often were not appropriately analyzed through multivariate modelling. In particular, some analyses contained few studies (e g, OCD in BD I, three studies), and in such instances, the publication bias tests used may be insensitive to potential publication bias.
The main strength of this review is being systematic and including the entire scientific evidence published so far in the main medical databases. The strength of the selected studies was that the diagnosis of BD and OCD were consistently based on the DSM criteria and were established by trained investigators using validated assessment scales mainly with inter-rater reliability (IRR).