1. Background
Bipolar disorder (BD) is characterized by fluctuation and relapses of mania and depression periods (1). In bipolar I disorder (BP-I), there is single manic episode or recurrent episodes of mania with or without episode of depression (2). Genetic background and environment are involved in the pathogenesis of BD.
Neurotrophins such as brain-derived neurotrophic factor (BDNF) have a critical role in the development and maintenance of the mammalian central nervous system (CNS) (3). BDNF is expressed throughout the brain, particularly in the hippocampus and prefrontal cortex (PFC). This neurotrophic factor has long-term effects on neuronal survival, migration, and growth (4). BDNF has a modulatory effect on cholinergic, dopaminergic, and serotonergic neurons; thus, it seems to involve in the etiopathogenesis of mental disorders (5).
The BDNF gene is located in the chromosome 11p13 (6). The gene of BDNF encodes an activity-dependent endogenous neurotrophin that might enhance the growth and function of 5-HT neurons in the brain. The functional polymorphism of BDNF G196A (Val66Met, rs6265) in the coding region of the BDNF gene results in a valine (Val) to methionine (Met) substitution in the 5 prodomain of the human BDNF protein (6). The polymorphism of Val66Met in the gene of BDNF impairs BDNF trafficking and secretion (7). In vivo studies reported an effect of the functional polymorphism of BDNF Val66Met on the glutamate system in human hippocampus (5).
There are inconsistent reports related to the role of BDNF Val66Met in susceptibility to BD (6-13).
The promoter insertion/deletion polymorphism in the serotonin (5-hydroxytriptamine (5-HT)) transporter (5-HTT) gene-linked polymorphic region (5-HTTLPR) consists of either 14 (short or S allele) or 16 (long or L allele) comprising 44-base pair repeat (2). Previously, we reported that the 5-HTTLPR might be a risk factor for susceptibility to BP-I among Kurdish population in Western Iran (2).
According to literature, there is no available study to examine the synergism between BDNF Val66Met and HTTLPR L/S variants in susceptibility to bipolar disorder.
2. Objectives
The aim of the present study was to investigate the role of BDNF Val66Met in susceptibility to BP-I in a population with Kurdish ethnic background in Western Iran. Also, we aimed to examine the synergism between BDNF Val66Met polymorphism and HTTLPR L/S variants in the risk of BP-I in our studied population.
3. Materials and Methods
3.1. Patients and Controls
The patient group consisted of 153 in- and out-patients with bipolar I disorder with the mean age of 35.5 ± 12.3 years including 76 females and 77 males. Normal IQ and suffering from BID according to DSM-IV-TR criteria were the inclusion criteria for studying the patient group. Patients who met the criteria for schizoaffective or schizophrenia or mental retardation and patients with medical condition were excluded from the study. All patients were selected in consecutive sampling method and after enough evaluation by psychiatrist and psychologist for inclusion and exclusion criteria, they were recruited in the study. Seven patients with psychotic symptoms more than 2 weeks in the absence of a major mood episode (suspicious to schizoaffective disorder), 2 patients with comorbid mental retardation and 2 patients with medical condition [hepatitis and chronic obstructive pulmonary disorder (COPD)] were excluded from the study. BID was previously confirmed in all patients by clinical interviews. Also, all patients were reevaluated by psychiatric interview and the SCID (structured clinical interview for DSM-IV-TR) was completed for all the participants. The patients were admitted to the psychiatry clinic of the Farabi hospital affiliated to Kermanshah University of Medical Sciences. A positive family history of major mood disorder (at least one first-degree relative suffering from bipolar I disorder, bipolar II disorder, or major depressive disorder) existed in 36 patients (24%). Nineteen patients (12.5%) were substance (opiate) dependent. Patients were treated with lithium, carbamazepine, and valproic acid (2). A review of medical records and a semi-structured interview were used to find a history of suicide attempt in patients (14). According to violent suicide methods (Hanging, profound cutting, and jumping) or non-violent suicide methods (taking poison or pills), violent and non-violent suicides were characterized (15).
The control group consisted of 146 healthy individuals including 63 females and 83 males with the mean age of 35.4 ± 10.9 years. The controls were age-matched with patients (P = 0.98). The controls were medical students, staff of medical school, Imam-Reza hospital, and central laboratory of Kermanshah University of Medical Sciences. All the controls were evaluated by psychiatric interview and the SCID was completed for all of them. They were healthy individuals without mental illnesses such as affective disorder, schizophrenia, anxiety disorder, personality disorder, and substance use disorders, and without a family history of psychiatric disorder among their first-degree relatives. According to the questionnaire that asked the participants about their ethnic background, those individuals that were from Kermanshah province in Western Iran with Kurdish ethnic background were selected for the study.
Written informed consent was obtained from each individual before participation in the study. The study was approved by the ethics committee of Kermanshah University of Medical Sciences and was in accordance with the principles of the declaration of Helsinki II.
3.2. Genotype Analysis
Ethylenediaminetetraacetic acid (EDTA)-treated whole blood that was collected from patients and controls was stored in a freezer (-20°C) for about three months. Genomic DNA was extracted from EDTA-treated whole blood using the phenol-chloroform method (16). Nanodrop spectrophotometer (Thermo) was used for the analysis of concentration and purity of extracted DNA. After DNA extraction from whole blood, aliquots from stock DNA were prepared and stored in a refrigerator (2°C). Experiments were performed during at least two months.
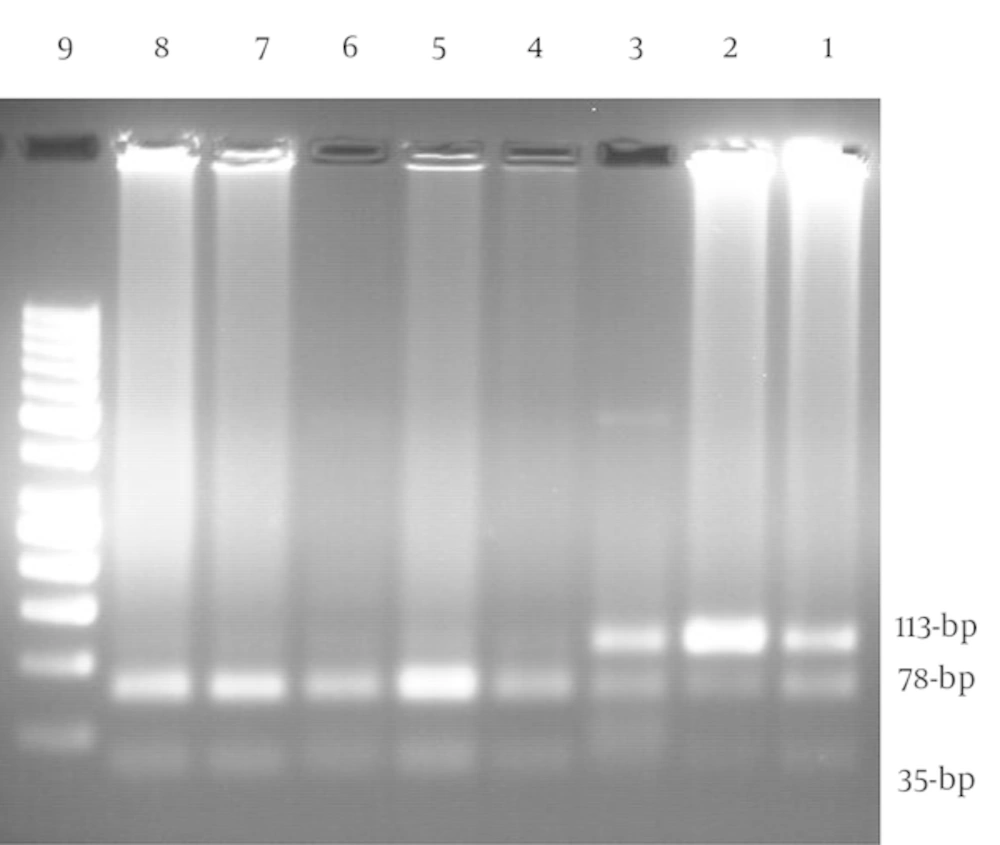
The BDNF G196A (Val66Met) genotypes were detected using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method by the following primers of forward: 5'-GAG GCT TGA CAT CAT TGG CT-3' and reverse: 5'- CGT GTA CAA GTC TGC GTC CT-3' (NCBI reference sequence: NC_000011.10). The primers used for amplification of the gene were those described by Grzywacz et al. (3). The PCR reagents consisted of 20 pmol of each primer, 2 μL DNA, 200 μM dNTPs, 1.5 mM MgCl2, 1 U Taq polymerase, and 2.5 μL of 10X PCR buffer with final volume of 25 μL. The PCR thermal cycling parameters were 1 cycle at 94°C for 5 minutes, 35 cycles at 94°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute, followed by final extension for 10 minutes at 72°C. The PCR machine of Eppendorf Master Cycler pro S (Germany) was used for amplification of target DNA. Five micro-litters of PCR products were monitored using electrophoresis on 1% agarose gel containing red gel stain that visualized under UV gel documentation (Quantum ST4). A negative control was amplified along with the samples to confirm the absence of contamination of PCR products. Amplified DNA fragments were digested overnight with the Eco72 I restriction endonuclease. The size of undigested allele A was 113-bp while the allele G was digested and dissociated into two fragments of 78- and 35-bp (Figure 1) (3).
The 5-HTTLPR genotypes were detected using PCR with the commercial forward primer of 5'-GGC GTT GCC GCT CTG AAT GC-3' and the reverse primer of 5'-GAG GGA CTG AGC TGG ACA ACC AC-3' (NCBI reference sequence: NC_000017.11), as previously described (2). The PCR reagents were used for the amplification of the BDNF gene and the PCR program was 1 cycle at 94°C for 5 minutes, 35 cycles at 94°C for 1 minute, 64°C for 1 minute, and 72°C for 1 minute, followed by final extension for 10 min at 72°C.
3.3. Statistics
The allelic frequencies were calculated by the chromosome counting method. Hardy-Weinberg equilibrium is mainly used to calculate the expected frequency assuming the absence of mutations, the absence of gene transfer, random mating, large population, and no selection. Genotype equilibrium was tested using the Hardy-Weinberg method for genotype distributions of BDNF Val66Met and 5-HTTLPR L/S polymorphisms to determine if they were consistent with Hardy-Weinberg equilibrium. Hardy-Weinberg equilibrium was assessed using chi-square test through the web site of www.OEGE.org/software.
The degrees of significance of differences in genotype and allele frequencies of BDNF and 5-HTTLPR between patients and controls were calculated using χ2 test. Odds ratios (OR) were calculated as estimate of relative risk for the disease and 95% confidence intervals (CI) were obtained by SPSS logistic regression. Two-tailed Student’s t-test analysis was also used to compare quantitative data. The categorical variables among groups were compared using χ2 test. Statistical significance was assumed at the P < 0.05 level. The Statistical package for the social sciences (SPSS) logistic regression (SPSS Inc., Chicago, IL, USA) version 16.0 was used for the statistical analysis.
4. Results
The characteristics of BP-I patients and healthy individuals are summarized in Table 1. Distribution of BDNF Val66Met genotypes was in Hardy-Weinberg equilibrium for both patients and controls (χ2 = 0.02, χ2 = 1.36, P > 0.1).
| Variable | Patients (n = 153) | Controls, (n = 146) |
|---|---|---|
| Gender | ||
| Female | 76 (49.7) | 63 (43.2) |
| Male | 77 (50.3) | 83 (56.8) |
| P Value | 0.09 | |
| Age, y | 35.5 ± 12.3 | 35.4 ± 10.9 |
| Range | 16 - 73 | 18 - 68 |
| P Value | 0.98 | |
| Family history of BP-I | - | |
| Absence | 114 (76) | |
| Presence | 36 (24) | |
| Hospitalization | - | |
| Once | 29 (20.1) | |
| Two or more | 115 (79.9) | |
| Suicide history | - | |
| Absence | 128 (85.3) | |
| Presence | 22 (14.7) | |
| Suicide history | ||
| Female | ||
| Absence | 64 (84.2) | |
| Presence | 12 (15.8) | |
| Male | ||
| Absence | 66 (86.8) | |
| Presence | 10 (13.2) | |
| Suicide method | - | |
| Violence | 8 (36.4) | |
| Non violence | 14 (63.6) | |
Abbreviation: BID, bipolar I disorder.
aValues are expressed as No. (%) or mean ± SD.
4.1. BDNF Variants
The BDNF G196A polymorphism was detected in 153 patients and 146 controls. In Table 2, the frequency of BDNF genotypes and alleles is demonstrated. The frequencies of GG (Val/Val), GA (Val/Met), and AA (Met/Met) genotypes in patients were 68, 28.8, and 3.2%, respectively, and in controls were 65.8, 28.8, and 5.4%, respectively (P = 0.63). Also, the frequency of BDNF A (Met) allele in patients (17.7%) was slightly lower than that in controls (19.9%, P = 0.5). Although the absolute percentage in the patient group was lower than that of the control group, the difference was not significant.
| Patients (n = 153) | Controls (n = 146) | χ2 | P Value | |
|---|---|---|---|---|
| BDNF | 0.89 | 0.63 | ||
| GG (Val/Val) | 104 (68) | 96 (65.8) | ||
| GA (Val/Met) | 44 (28.8) | 42 (28.8) | ||
| AA (Met/Met) | 5 (3.2) | 8 (5.4) | ||
| BDNF alleles | 0.45 | 0.5 | ||
| G (Val) | 252 (82.3) | 234 (80.1) | ||
| A (Met) | 54 (17.7) | 58 (19.9) |
aValues are expressed as No. (%).
4.2. Interaction Between BDNF and 5-HTTLPR Alleles
Previously, we reported that 5-HTTLPR insertion/deletion polymorphism might be involved in susceptibility to BP-I (2). In the present study, we examined synergism between BDNF G196A (Val66Met) and 5-HTTLPR L/S alleles. As depicted in Table 3, the concomitant presence of BDNF G (Val) and 5-HTTLPR S alleles tended to increase the risk of BP-I by 1.41 times (P = 0.064) compared to the combined presence of BDNF G and 5-HTTLPR L alleles. Also, the concomitant presence of BDNF G (Val) and 5-HTTLPR S alleles tended to enhance the risk of BP-I by 1.28-fold (P = 0.062) compared to the concomitant presence of BDNF A and 5-HTTLPR S alleles (Table 3).
| BDNF | HTTLPR | Patients [OR (95% CI, p)] | Control Group |
|---|---|---|---|
| G | L | 110 (36.5) Reference group | 114 (42.5) Reference group |
| A | L | 15 (5) | 8 (3) |
| χ2 = 2.16, P = 0.14 | |||
| 0.72 (0.46 - 1.12, 0.14) | |||
| G | S | 139 (46.2) | 102 (38.1) |
| χ2 = 3.4, P = 0.064b | |||
| 1.41 (0.97-2.04, 0.064) | |||
| χ2 = 3.5, P = 0.061c | |||
| 1.28 (0.98 - 1.64, 0.062) | |||
| A | S | 37 (12.3) | 44 (16.4) |
| χ2 = 0.28, P = 0.59 | |||
| 0.95 (0.8 - 1.13, 0.59) |
aOverall χ2 = 6.59, P = 0.08 comparing four allele groups of BDNF G/HTTLPR L, BDNF G/HTTLPR S, BDNF A/HTTLPR L, BDNF A/HTTLPR S.
bCompared to BDNF G/HTTLPR L.
cCompared to BDNF A/HTTLPR S.
No association was detected between BDNF genotypes (P = 0.65) and suicide attempt (data not shown).
5. Discussion
BDNF is a vital trophic protein for neuronal survival and differentiation in the developing nervous system (5). The findings of the present study did not demonstrate an association between BDNF Val66Met with the risk of BP-I among Kurdish population in Western Iran.
There are inconsistent reports related to the role of BDNF Val66Met in susceptibility to BD. Lack of association between BDNF Val66Met and BD among bipolar patients has been reported by Brazil (7) and Switzerland (8). Also, among Caucasians (Whites from United Kingdom) the BDNF Val66Met was not associated with BP-I and BP-II (6). Further, among Han Chinese population from Taiwan, the genotype of BDNF Val/Val was associated with BP-II but not with BP-I that might indicate the distinct pathogenesis of BP-I and BP-II (9). Different etiology and characteristics of BP-I and BP-II during the course of disease were confirmed by Lee et al. (10) that reported the association of the BDNF Val66Met with BP-II but not with BP-I among Han Chinese population in Taiwan. They suggested the two types of disorder that might be genetically distinct (10). Furthermore, another study among Han Chinese population of Taiwan confirmed that the BDNF Val allele was not associated with BP-I, while this allele was associated with anxiety disorder comorbidity in BP-I (11). However, Lohoff et al. reported a positive association between the Val allele of BDNF and BP-I in patients of European descent (12). Recent meta-analysis by Wang et al. (13) including a total of 7219 patients and 9832 controls of Caucasians and Asians reported the absence of a significant association between the Val66Met polymorphism and susceptibility to BD in all studied individuals. However, there was a trend towards significant association between this polymorphism and BD in Caucasian population (13). It seems the BDNF Val66Met might not be associated with the risk of BD in Asian population. The reason for inconsistent results might be due to different frequencies of Val and Met alleles of BDNF Val66Met among various ethnic groups so that a frequency of about 80% for Val allele among the European populations was observed while in Asian populations this frequency was only 50% (11). Also, clinical heterogeneity of bipolar disorder and the absence of differentiation between BP-I and BP-II groups with different etiologies and pathogenesis and also gene-gene interactions are the other possible reasons for obtained conflicting results (9).
BDNF regulates serotonin systems and the survival of serotonin neurons (10). In our previous report, we suggested that 5-HTTLPR might be associated with the risk of BP-I among Kurdish population in Western Iran (2). In the present study, we examined the interaction between BDNF variants and 5-HTTLPR L/S polymorphism and found a synergism between BDNF G (Val) allele and 5-HTTLPR S allele that tended to increase the risk of BP-I by 1.28-fold compared to the concomitant presence of BDNF A and 5-HTTLPR S alleles. Also, the combined presence of BDNF G (Val) and 5-HTTLPR S alleles compared to combined presence of BDNF G and 5-HTTLPR L alleles tended to enhance the risk of BP-I by 1.41 times in our population.
To our knowledge, there is no study to examine epistatic interaction between BDNF Val66Met and 5-HTTLPR L/S alleles and its influence on the risk of BP-I.
There is an evidence of interaction between serotonin system and BDNF, especially in neurogenesis and synaptic plasticity (17). Serotonin not only acts as neurotransmitter, but also regulates cell survival and synaptogenesis. There are some evidence that the action of BDNF as an antidepressant is through the modulation of serotonergic functions (18). In the study of Grabe et al. (17), the presence of 5-HTTLPR SS genotype was associated with an increased risk of depression only in concomitant presence with BDNF Val/Val genotype (17). The structural and functional impact of the 5-HTTLPR SS genotype on limbic brain areas like the amygdale and perigenual cingulate cortex has not been observed in carriers of 5-HTTLPR S allele combined with BDNF Met allele (17). However, in the study of Kudinova et al. (19), the greatest risk of depression has been observed among women with a history of child abuse in the presence of at least one copy of the 5-HTTLPR S allele and BDNF Met allele. There is an evidence that serotonin enhances the expression of BDNF. However, the S allele of 5-HTTLPR allele is associated with decreased some exons of BDNF in the hippocampus and prefrontal cortex (PFC) (18). Also, the expression of BDNF gene has significantly decreased in leukocytes of healthy individuals’ carrier of the 5-HTTLPR S allele (17). Thus, the S allele of 5-HTTLPR might be involved in lifelong epigenetic changes that may result in functional alterations with decreased structural and functional plasticity of BDNF, predisposing individuals to depressive disorders (18).
5.1. Conclusions
Our study was conducted for the first time on Kurdish population in Western Iran and indicated the absence of any association between BDNF Val66Met and BP-I. Although the interaction between BDNF G (Val) allele and 5-HTTLPR S allele did not reach to a statistical significance, the presence of both alleles tended to increase the risk of BP-I compared to the concomitant presence of BDNF A and 5-HTTLPR S alleles. Also, the combined presence of BDNF G (Val) allele and 5-HTTLPR S allele compared to the combined presence of BDNF G and 5-HTTLPR L alleles might tended to enhance the risk of BP-I in our population.
5.2. Limitations
The lower number of individuals with concomitant presence of some BDNF and 5-HTTLPR variants is the limitation of the study.