1. Background
Schizophrenia is a clinical syndrome with variable psychopathology, but is a profoundly destructive condition affecting cognition, emotion, perception and behavior (1). These symptoms are variable in different patients and over time, whereas the impact of the disease is invariably intense and often long-lasting (1). The incidence of schizophrenia during a person’s life ranges from 0.2% to 0.7%. Based on the criteria of Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) (2), the annual incidence ranges from 0.5 to 5% per 10 000 individuals (3).
Given that antipsychotic treatment in some patients does not alter the course of disease or prevent dysfunction, alternative procedures or concurrent adoption of other treatments is crucial (4). Evidence suggests that the pathophysiology of schizophrenia is associated with immune system dysfunction and cytokines play a role in incidence of schizophrenia (5). Impaired immune responses are associated with inflammation in the central nervous system (CNS). Moreover, immune response impairment type I is an associated with reduced activity of key enzymes of tryptophan metabolism, indoleamine 2 and dioxygenase 3 (IDO). Lower IDO activity results in higher production of kynurenic acid, n-methyl d-aspartate antagonist. Hence, glutamatergic neurotransmitters are reduced in patients with schizophrenia. Inflammatory phase of schizophrenia is associated with increased production of prostaglandin E2 and increased secretion of cyclooxygenase -2 (COX-2) (6). Like celecoxib, COX-2 inhibitors have a beneficial effect on the treatment of inflammatory phase of schizophrenia, particularly in the early stages of schizophrenia (6). Furthermore, aspirin inhibits N-methyl D-aspartate receptor as an anti-inflammatory drug, preventing some of the symptoms of schizophrenia associated with inflammation (7).
2. Objectives
Due to early onset of schizophrenia in most cases, hospital care, rehabilitation and support services, cultural, economic and social damages caused by schizophrenia surpass the sum of losses incurred by all cancers combined. Moreover, the complexity of schizophrenia makes it difficult for a single therapeutic approach to deal with this multifaceted disorder. Therefore, it seemed critical to study the disease and its treatment. According to suggested inflammatory pathophysiology of schizophrenia, our hypothesis was that anti-inflammatory agents such as aspirin could reduce the symptoms of this disorder.
3. Materials and Methods
This was a triple blind, prospective clinical trial study that was conducted at Noor Hospital of Isfahan University of Medical Science in Isfahan city from January 2015 for one year. The population included patients with schizophrenia based on clinical interview and DSM-IV-TR criteria (2) within the age group of 18 to 65 years old referred to Noor Hospital psychiatric clinic or emergency. The purpose of this project, methods and frequency of follow-up were explained to the patients or their legal guardians. The subjects participated after providing a written consent. The inclusion criteria were age of 18 to 65 years old, diagnosis of schizophrenia based on clinical interview and DSM-IV-TR, and having a two-year history since the onset of the disease. The exclusion criteria were unwillingness to participate in the study, failure to see the doctor for follow-up for whatever reason, unstable medical illness and medical history, contraindications for use of aspirin, such as asthma or seasonal allergies, ulcers, kidney disease, active bleeding or clotting of blood disorders such as hemophilia or bleeding, gout, nasal polyps, chronic use of Non-steroidal Anti-Inflammatory Drugs (NSAID), concomitant use of corticosteroids for any reason and maternity (8).
The sample size was calculated to be 20 subjects per group. This sample size was calculated based on the results of previous studies (8-10) by assuming a test power of 80% and a confidence level of 95% and using the following Formula 1:

Where:
n = sample size,
Z1-α/2 = 1.96 when α = 5% for two-sided test,
Z1-β = 0.842 when β = 20% (test power = 80%),
P = probability of the main outcome.
The patients, through a number table, were randomly divided to three groups of 20 members labeled intervention 1, intervention 2 and control. Patients in each group were initially evaluated through the positive and negative syndrome scale (PANSS) scale (9) in terms of severity of schizophrenia symptoms. The PANSS is a medical scale used for measuring symptom severity of patients with schizophrenia; published in 1987 by Stanley Kay, Lewis Opler and Abraham Fiszbein. It is widely used in the study of antipsychotic therapy. The name refers to two types of symptoms in schizophrenia, as defined by the American Psychiatric Association: positive symptoms, which refer to an excess or distortion of normal functions (e.g. hallucinations and delusions), and negative symptoms, which represent a diminution or loss of normal functions. The PANSS is a relatively brief interview, requiring 45 to 50 minutes to administer and has validity of 80% (9).
In this clinical trial, stratified randomization was used with the baseline schizophrenia symptoms of the participants. For this, all participants were divided to three groups; those reciving the placebo, those reciving aspirin 325 mg+antipsychotic, and those reciving aspirin 500 mg+antipsychotic. Then, the participants in each of the groups were assigned randomly to receive drugs on a 1: 1: 1 ratio. Randomization was done by one of the researchers, who did not have a role in the treatment of the participants. The randomization sequence was computer-generated, with the randomization itself conducted through SPSS 20 software (SPSS, Inc., Chicago, IL, USA) (random number generation). We used a variable block size of four and six for randomized sequence generation. Also, allocation concealment was done by the researcher, who was responsible for the randomization. For this purpose, the numbered envelopes that contained the name of the drugs (placebo, aspirin 325 and aspirin 500) were used. After being allocated randomly to the groups, all participants were referred to the hospital’s pharmacy to obtain their drugs. The outcomes of the study were recorded by the psychiatrist of the psychiatry ward, who made no other contribution to the study. A resident of psychiatry generated the random allocation sequence and enrolled participants, and a co-worker psychologist assigned participants to the intervention.
The authors confirm that all ongoing and related trials for this intervention have been registered. Indeed, in this study, for ethical considerations, the participants or their guardian were informed about the objectives and nature of the study, and each participant or their guardian provided a written consent in their native language (Persian) prior to the study. Also, we were committed to keep all of the participants’ information confidential. All patients were in the acute phase of the disorder and the sample was homogenous. In this study, we did not investigate the cutoff point of symptoms scores, but we compared the severity of symptoms before and after the intervention between groups.
At baseline, clinical evaluation involved cell count, liver function, kidney, thyroid and cardiac function tests. Moreover, cardiac status was assessed through routine electrocardiogram (ECG). The patients were then referred to a psychiatrist, who prescribed their medication. A professor of psychiatry and resident interviewed the participants and filled the questionnaires.
In addition to antipsychotic medication (equivalent to 100 mg chlorpromazine), the intervention 1 group received daily aspirin produced by Daroupakhsh Co. for six weeks at a dose of 325 mg, while intervention 2, in addition to antipsychotic medication, received daily aspirin with a dose of 500 mg for six weeks. Previous studies focused on the impact of aspirin at a dose of 1000 mg on the treatment of schizophrenia (10). Hence, the current study applied high-dosage aspirin to reduce the cardiovascular complications caused by schizophrenia, while the lower dosages (i.e. 325 mg and 500 mg) were administered to reduce gastrointestinal complications.
From 60 participants, 18 had received olanzapine, four had received haloperidol, 35 had received risperidone and three had received chlorpromazine. The antipsychotics administered for all patients were equal to 100 mg of chlorpromazine.
The control, in addition to antipsychotic medication, received daily placebo for six weeks. It should be noted that the placebo tablets had the same shape and color of the effective aspirin. Drug and placebo were coded A, B and C. In order to reduce the gastrointestinal side effects, omeprazole 20 mg daily was given to each patient. Neither the examiner nor the clinician and the patient were aware of the drug compounds since they were labeled A, B and C. The questionnaire was confidential and filled based on codes, made available only upon the request of the patient. Antipsychotic dose was fixed from the beginning to the end of the study and participants did not take any additional doses of antipsychotics.
The PANSS scale was administered on the first day, the end of the sixth week and one month after cessation of aspirin or placebo, followed by recording and comparing the scores. The data were analyzed through SPSS 20, and based on descriptive statistics of frequency distribution, mean and standard deviation. Moreover, the results of the test were compared through repeated measure analysis of covariance (ANOCOVA) and logistic regression. After data analysis, it was revealed that A was the control group, B was intervention 1, and C was intervention 2. Drug side effects (aspirin or antipsychotics) were not observed in any participants.
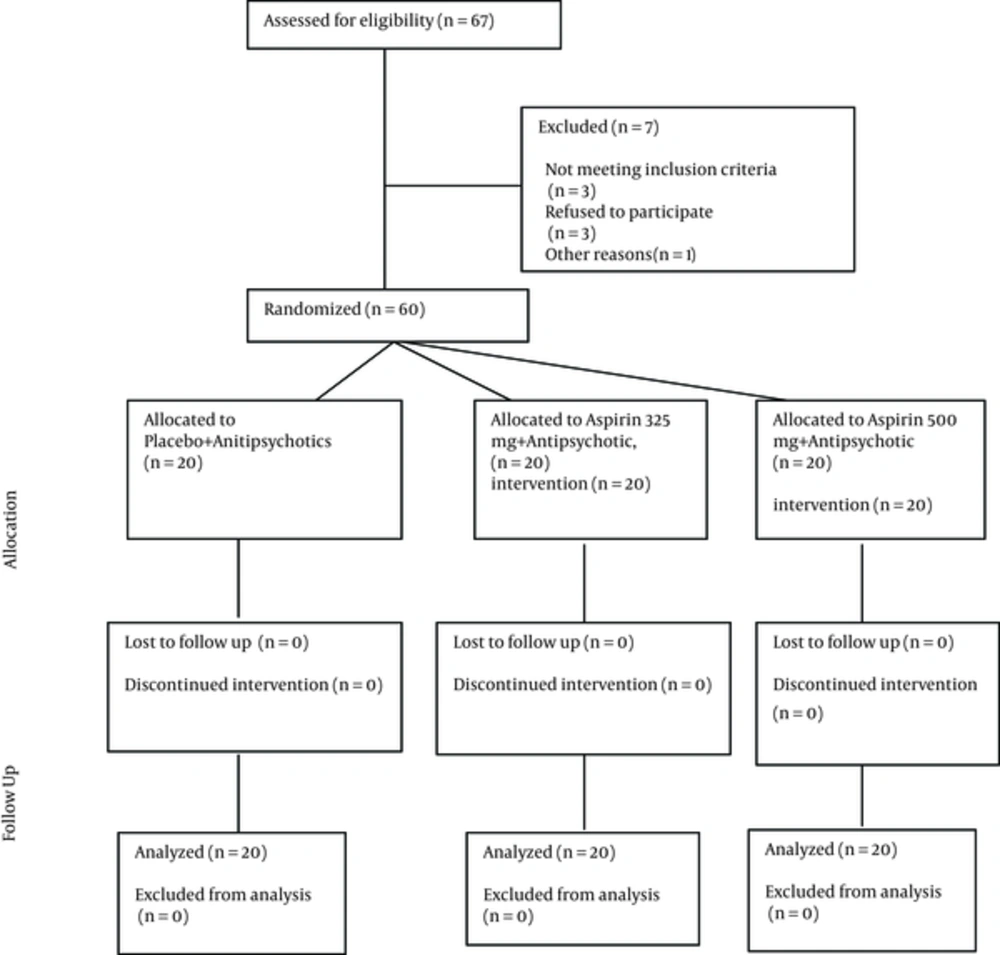
The protocol was approved by the institutional review board (IRB) of Isfahan University of Medical Sciences and carried out in agreement with the declaration of Helsinki and its subsequent revisions. After complete explanation of the study details, written informed consent was obtained from eligible patients and their legally authorized representatives, informing the participants of their rights to withdraw from the trial at any time without any interruption in their healthcare benefits. This trial was registered at the Iranian clinical trials registry (IRCT201211211556N47; www.irct.ir). The CONSORT diagram shows the flow of participants through each stage of the randomized trial.
4. Results
The blind groups were compared in terms of age, gender, marital status, education, occupation and family history of the disease (Table 1). The results showed that none of the variables in the three groups had a significant difference (P < 0.05).
| Group Variable | A | B | C | Pb |
|---|---|---|---|---|
| Gender | 0.942 | |||
| Male | 13 (65) | 20 (60) | 14 (70) | |
| Female | 7 (35) | 8 (40) | 6 (30) | |
| Marital status | 0.863 | |||
| Single | 15 (75) | 14 (70) | 15 (75) | |
| Married | 4 (20) | 6 (30) | 4 (20) | |
| Divorced | 1 (5) | 0 | 1 (5) | |
| Education level | 0.683 | |||
| Under diploma | 6 (30) | 9 (45) | 4 (20) | |
| Diploma | 10 (50) | 7 (35) | 11 (55) | |
| Associate’s degree | 2 (10) | 3 (15) | 4 (20) | |
| MS and higher | 2 (10) | 1 (5) | 1 (5) | |
| Occupation | 0.368 | |||
| Unemployed | 13 (65) | 13 (65) | 8 (40) | |
| Housewife | 2 (10) | 5 (25) | 6 (30) | |
| Clerk | 1 (5) | 0 | 1 (5) | |
| Self-employment | 4 (20) | 2 (10) | 5 (25) | |
| Family history of disease | 0.552 | |||
| Yes | 6 (30) | 3 (15) | 3 (15) | |
| No | 14 (70) | 17 (85) | 17 (85) | |
| Age, mean ± SD | 31.35 ± 6.78 | 33.5 ± 10.04 | 33.45 ± 5.81 | 0.664 |
Abbreviations: A, placebo + antipsychotics; B, aspirin 325 mg + antipsychotic; C, aspirin 500 mg + antipsychotic.
aValues are expressed as No. (%).
bRepeated measure ANOVA.
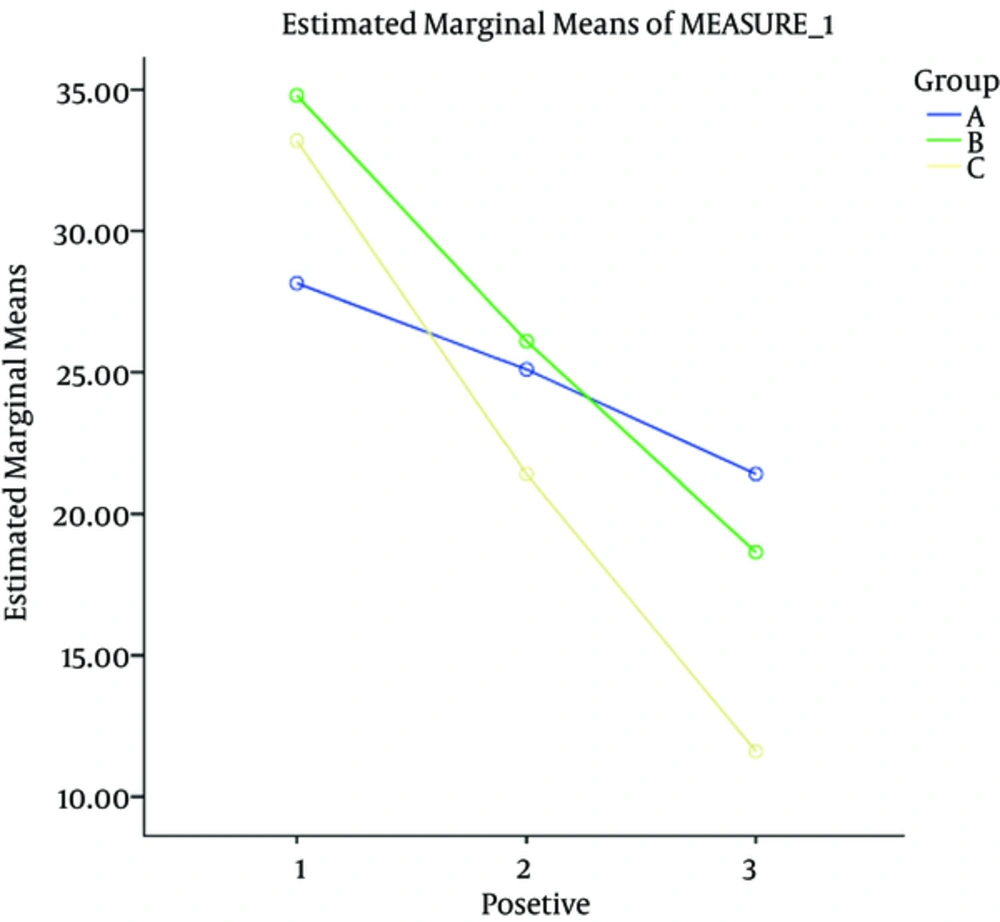
The baseline value (positive symptoms, negative symptoms, general psychopathology and the final score) was compared at the beginning of the study in three groups, according to the test. The results indicated that all variables evaluated in the three groups were significantly different (P < 0.05)
| Group Variable | A | B | C | P |
|---|---|---|---|---|
| Positive symptoms | 18.15 ± 7.21 | 34.80 ± 6.98 | 33.20 ± 7.02 | 0.012 |
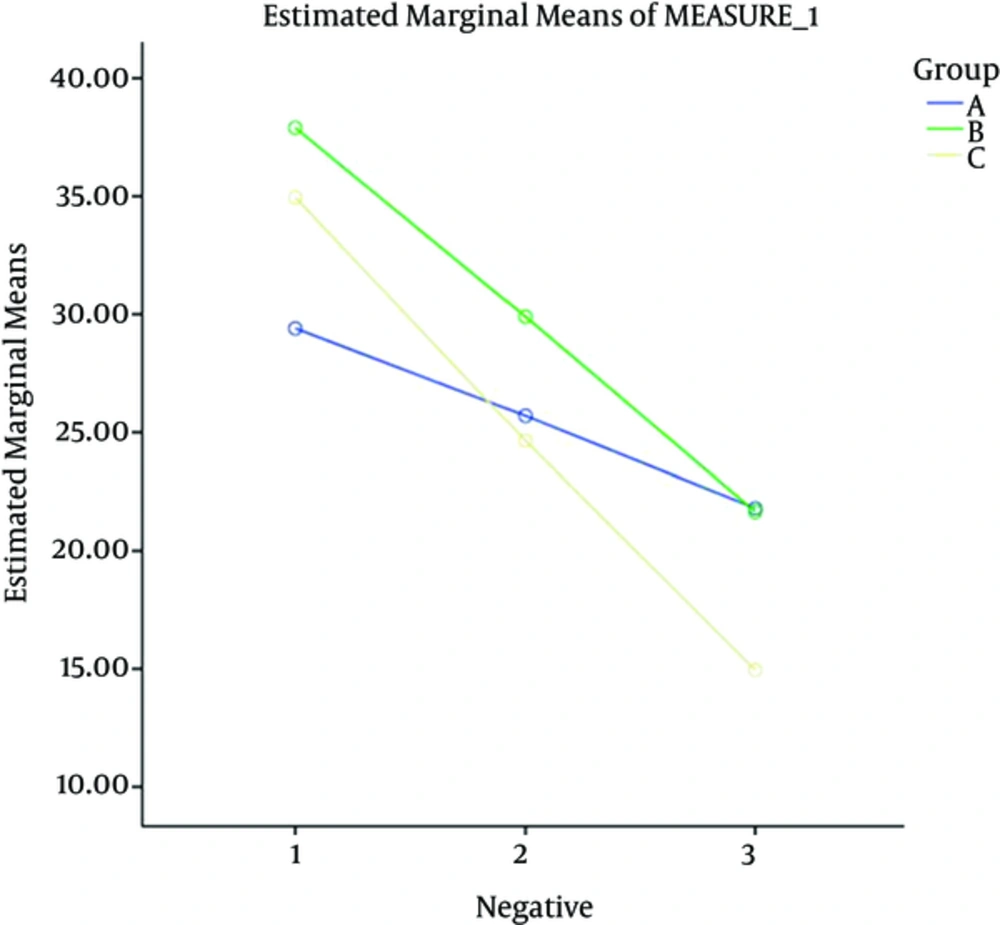
| Negative symptoms | 29.40 ± 9.77 | 37.90 ± 8.00 | 34.95 ± 9.83 | 0.017 |
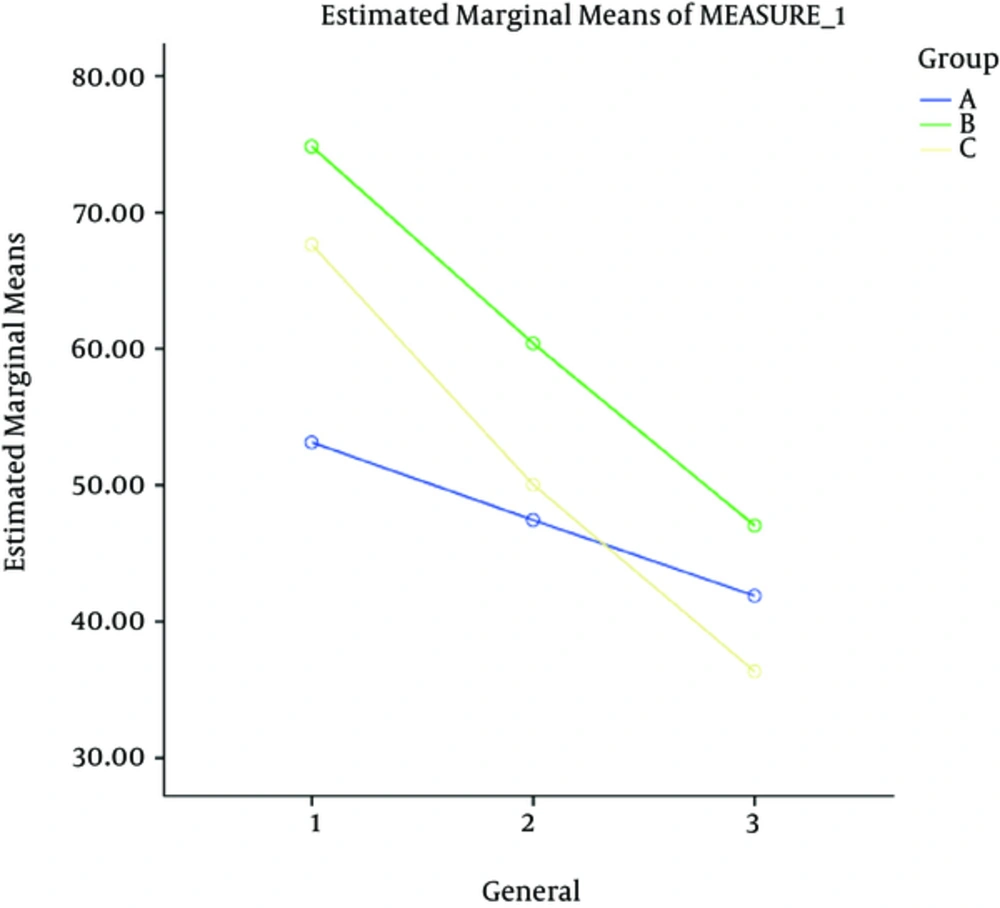
| General psychopathology | 53.15 ± 12.63 | 74.85 ± 15.85 | 67.65 ± 21.41 | 0.001 |
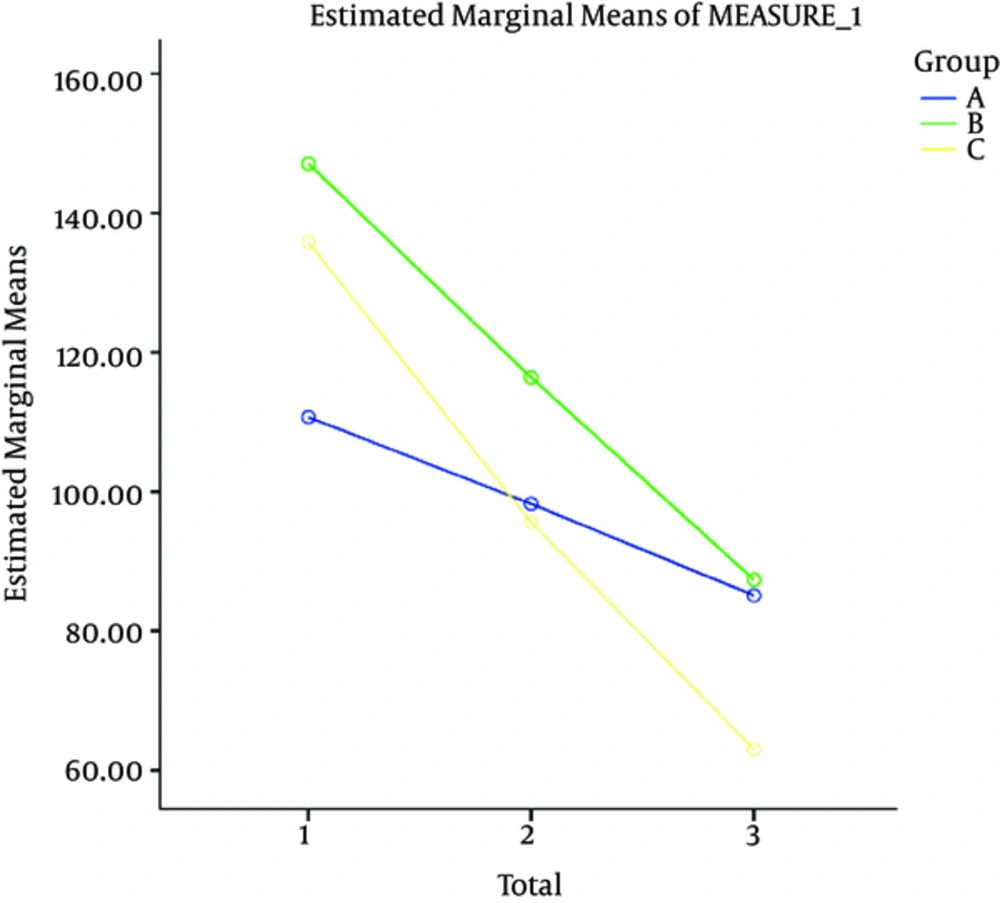
| Final score | 110.70 ± 24.32 | 147.10 ± 27.76 | 135.80 ± 35.5 | 0.001 |
aValues are expressed as mean ± SD.
Abbreviations: A, control; B, aspirin 325 mg + antipsychotic; C, aspirin 500 mg + antipsychotic.
aValues are expressed as mean ± SD.
bRepeated measure ANOVA.
At the next stage, each of the dependent variables (positive symptoms, negative symptoms, general psychopathology and the final score) was compared during the intervention period in the three groups using repeated measures. The difference in the three groups at baseline was adjusted by considering the values as confounding variables and controlling their effects.
Abbreviations: A, control; B, aspirin 325 mg + antipsychotic; C, aspirin 500 mg + antipsychotic.
aValues are expressed as mean ± SD.
bRepeated measure ANOVA.
Abbreviations: A, control; B, aspirin 325 mg + antipsychotic; C, aspirin 500 mg + antipsychotic.
aValues are expressed as mean ± SD.
bRepeated measure ANOVA.
Abbreviations: A, control; B, aspirin 325 mg + antipsychotic; C, aspirin 500 mg + antipsychotic.
aValues are expressed as mean ± SD.
bRepeated measure ANOVA.
The results indicated that the effect of the intervention on changes during intervention was significant. In order compare the two groups in a pairwise manner, a follow-up test was conducted. The results showed that all three groups were significantly different (P < 0.05).
In order to compare the two groups in a pairwise manner, the follow-up LSD test was conducted. The results showed that all three groups were significantly different (P < 0.05).
Generally, the examinations showed that the intervention effect was greater on the final outcome and positive and negative symptoms and general psychopathology in four weeks after discontinuation of the intervention compared to the other time points.
5. Discussion
The aim of our study was to evaluate the effect of adding aspirin to antipsychotic treatment on severity of symptoms in schizophrenia patients. The results indicated that the effect of the aspirin on changes during intervention was significant and all three groups were significantly different (P < 0.05).
Also results showed that the aspirin usage effect was greater on the final outcome and positive and negative symptoms and general psychopathology in four weeks after discontinuation of the intervention, compared to the other time points.
Regarding the effectiveness of aspirin in reducing the severity of positive, negative and general psychopathology of schizophrenia, results of our study reinforces the role of inflammation in the etiology of schizophrenia.
Previous studies suggested that inflammation has been proposed as the pathogenesis of schizophrenia. So far, several studies have focused on the impact of celecoxib and aspirin at a dose of 1000 mg on the treatment of schizophrenia. Hence, the current study applied high-dosage aspirin to reduce the cardiovascular complications caused by schizophrenia, while the lower dosages (i.e. 325 mg and 500 mg) were administered to reduce gastrointestinal complications. Furthermore, the six-week period was the minimum time required compared to other studies. The results showed that addition of aspirin to short-term treatment and one-month follow-up after stopping aspirin was effective in improving positive symptoms, negative symptoms and general psychopathology of schizophrenia patients (7, 10).
In a study conducted by laan et al. (10), 70 patients with schizophrenia and schizoaffective disorder were divided in a double-blind manner to two experimental and control groups. Patients with more than five years of illness were excluded. One group received a daily aspirin dosage of 1000 mg, while the other group received placebo for three months. Seventy percent of patients were given olanzapine, clozapine and risperidone as an antipsychotic drug. Moreover, a daily dosage of 40 mg of pantoprazole was given to prevent gastrointestinal problems. The positive and negative symptom scale (PANSS scale) was adopted to evaluate the patients. At the end, there was a significant reduction in the group receiving additional aspirin for treatment compared with the placebo. Aspirin did not lead to any significant cognitive impairment and there were insignificant complications observed.
In the recent years adjuvant therapy and polypharmacy for schizophrenia has been a major concern while being applied to several other chronic diseases. The results of this study indicate that aspirin, as an add-on to antipsychotic, has significant beneficial effect on the primary negative and psychopathologic symptoms of schizophrenia in males. Regarding the primary negative symptoms, we should consider factors like positive, extrapyramidal and depressive symptoms to be confounding to the changes occurring in negative symptoms (11, 12). Although the patients should be adequately stabilized before entering the trial, changes in positive, extrapyramidal and depressive symptoms were actually minimal. Therefore, it is safe to attribute improvement in negative symptoms to reduce primary negative symptoms.
In a study conducted by Sommer et al. (13), a meta-analysis of NSAID non-steroidal anti-inflammatory drug was carried out on schizophrenia patients. Because evidence suggests that inflammation plays a role in the pathogenesis of schizophrenia, it seemed inflammatory drugs such as NSAID (ibuprofen, naproxen, diclofenac and acetylsalicylic acid) were useful in reducing the severity of schizophrenia symptoms. The results were measured by PANSS and ultimately evaluated through a double-blind study involving 264 patients. In one study, celecoxib was used and in another acetylsalicylic acid was used. Finally, it was concluded that addition of NSAID to antipsychotic treatment curtailed the positive and negative symptoms in schizophrenia patients. Unlike celecoxib, which is associated with higher risk of cardiovascular disease, the addition of aspirin reduced cardiovascular episodes in patients with schizophrenia, even though it was accompanied with a risk of gastrointestinal bleeding. The results of these studies were consistent with our results.
Muller et al. at the University of Munich conducted a research involving inflammation in the pathogenesis of schizophrenia, where 49 patients with schizophrenia with less than two years of disease were examined. The experimental and control groups were selected through a double-blind, randomized procedure. The experimental group received celecoxib containing amisulpride. They received 200 -1000 mg of amisulpride and 400 mg of celecoxib as placebo. PANSS and CGI criteria were evaluated after six weeks. It was concluded that the addition of celecoxib in the treatment regimen was far more effective in the early stages compared with placebo in reducing positive and negative symptoms of schizophrenia. Moreover, it was the first time the relief of negative symptoms by celecoxib was observed in patients (14).
In 2007, Akhundzadeh et al. (15) at University of Tehran carried out a relevant study. Given that there is evidence that the pathophysiology of schizophrenia is associated with impaired immune system and cytokines are involved in its creation while cyclooxygenase -2 inhibitors are effective in reducing the production of inflammatory cytokines such as Th1, 60 chronic schizophrenia patients were examined for eight weeks. They were divided to two groups, half of the patients received 6 mg of risperidone daily with 400 mg of celecoxib (BID/200 mg), while the other group received the placebo. Risperidone combined with celecoxib compared with risperidone alone led to a dramatic reduction in PANSS positive and negative symptoms. Moreover, the extrapyramidal complications in the placebo group were higher than the group treated with celecoxib.
Given the role of immune system in the development of schizophrenia and increased levels of inflammatory cytokines in the CNS, Muller et al. (5) studied cyclooxygenase inhibitors, like Celecoxib-2, as an immune regulator drug, on 50 patients in the acute phase of schizophrenia, randomly divided to two experimental and control groups. The experimental group received 2 - 6 mg of risperidone daily with 400 mg of celecoxib, while the other group received 2 - 6 mg of risperidone daily plus placebo. There were no differences in age, gender, duration and severity of symptoms, psychopathology of the patient and the dose and plasma levels of risperidone between the two groups. After five weeks, the patients were evaluated by PANSS, and ultimately, the group receiving celecoxib along with risperidone showed a significant reduction in symptoms.
In 2006, Laan et al. (16) examined the effects of adding acetylsalicylic acid in the treatment of 80 patients with schizophrenia, schizophrenia form and schizoaffective. The patients were assigned randomly to experimental and control groups through a double-blind procedure. For three months, one group received regular antipsychotic with placebo while the other group was given 1000 mg of acetylsalicylic acid daily with pantoprazole to reduce gastrointestinal complications. After three months, the results were as follows: positive and negative symptoms were curtailed and cognitive performance improved in the control group.
Conclusively, it is desired that aspirin, as a non-steroid anti-inflammatory drug (NSAID), be able to provide patients with the benefits of anti-coagulant and anti-inflammatory in the brain without potential peripheral adverse effects. The results of this study should be interpreted with consideration of its limitations. Small sample size and short observation period are the major limitations of this study. These results may be confirmed in more prolonged trials with larger sample sizes to ensure study power. Also, it should be mentioned that PANSS is limited to assessing behavioral problems; therefore, patient cognitive function improvement is not measurable. In conclusion, this triple-blind, placebo-controlled clinical trial revealed aspirin as a tolerable adjunct to antipsychotics with improvement in primary positive, negative and general psychopathology symptoms of schizophrenia in patients. Additional studies with long-term follow-up periods seem to be essential to reinforce clinical use of adjuvant aspirin in schizophrenic patients.
5.1. Conclusions
Based on our results, we can conclude that aspirin can be equally useful in the reduction of positive, negative and general psychopathology of schizophrenia, but further studies are needed to compare the effectiveness of different types of usage of NSAIDs in schizophrenia. We purpose more studies for investigation of the effect of aspirin augmentation on reduction of schizophrenia symptoms.