1. Background
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental psychiatric condition, with a prevalence of 5 - 10% (1). It results from developmental dysfunctions of various brain areas, mainly the prefrontal cortex (2, 3). Stimulants are highly effective in controlling symptoms of ADHD in 75% of patients (4, 5), but 30% of individuals with ADHD do not respond to these medications or cannot tolerate their side effects (4). Thus, alternative treatment approaches need serious consideration.
Nootropics are psychoactive medications with stimulant-like effects (6). Piracetam is one of the nootropic medications (7) and can facilitate brain blood flow, cerebral oxygen bioavailability (8), and brain metabolism rate (9). Studies have shown that piracetam acts on neuronal membrane, increases synaptic neurotransmitter release, enhances neuron excitability, and stimulates the cerebral cortex (10). Piracetam has been studied in several cognitive and neurodevelopmental disabilities (11) and in different age ranges, and results have shown that it is well-tolerated, with almost no side effects or drug-drug interaction (12). Piracetam seems to improve alertness (11), attention span, and concentration (13), eye-hand coordination (11), memory (13), learning (11), and language function (13). Considering the mentioned documents, it can be hypothesized that piracetam may be effective in reducing symptoms of ADHD and could be an appropriate candidate for therapeutic purposes in this disorder.
There are only two published studies on the effect of piracetam in children with ADHD. These controlled open trials used piracetam as monotherapy at the doses of 40 - 70 mg/kg/day for 4 - 6 weeks in ADHD children aged 6 - 13 years. Both studies demonstrated the high effectiveness of piracetam in reducing ADHD symptoms and its superiority over placebo, with more response rate at higher doses (14, 15). Given the paucity of studies on the effect of piracetam on ADHD and their open design, which demonstrated positive outcomes, performing more precisely designed trials on greater sample sizes and wider age ranges seems necessary.
2. Objectives
The present study was performed to evaluate the short-term effects of piracetam as adjuvant therapy in children with ADHD who were under treatment of methylphenidate (MPH), using a randomized, double-blind, placebo-controlled design.
3. Methods
3.1. Subjects
Our sample consisted of 36 children of both genders who were admitted to outpatient child psychiatric clinics of Iran University of Medical Sciences (IUMS) during the second half of 2015.
Inclusion criteria were: (1) being 6 - 16 years old;(2) diagnosed as having ADHD by DSM-IV-TR criteria (16), the ADHD section of Child Symptom Inventory-DSM-IV-Version (CSI-4), Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (KSADS-PL), and the clinical judgment of child psychiatrist; (3) being drug-naïve; (4) good health on medical review of systems and complete routine physical and neurological examinations, and (5) having written informed consent from parents and assents from patients. The exclusion criteria were: (1) having any comorbid major mood, psychotic, neurologic, developmental, or medical disorders, or substance use (2) having an intellectual disability; and (3) being under any therapy during the study.
To include an ordinary group of patients with ADHD, children with common comorbidities of ADHD (18) were not excluded from the study. The study’s protocol was registered at the Iranian Registry of Clinical Trials (registration number: IRCT201303036923N2), approved by the Committee of Medical Ethics of IUMS (number: 19912), and granted by the Mental Health Research Center of IUMS (grant number: 19912-121-04-91).
3.2. Measurements
Subjects were evaluated by the following tests: (1) Kiddie Schedule for Affective Disorder and Schizophrenia, Present and Lifetime Version (KSADS-PL) (17) at baseline for diagnosing ADHD and other co-morbidities; (2) ADHD section of the Child Symptom Inventory-DSM-IV-Version (CSI-4) (18, 19) as diagnostic and severity assessment tool; 3) Conner’s Parents Rating Scale (CPRS-R) (20) to assess the severity of ADHD; (4) Clinical Global Impression-Improvement (CGI-I) scale (21) to assess the degree of improvement; (5) Children’s Global Assessment Scale (CGAS) (22) to estimate functional status; (6) complete medical review of systems, and complete physical and neurological examination (height, weight, pulse rate, and blood pressure); and (7) New York State Psychiatric Institute side effect form for the clinical trial in children and adolescents (23) for weekly assessment of medication adverse effects. KSADS-PL was completed at baseline. Other tools were used at baseline and after the third and sixth weeks of the study. All tests are validated, and their reliabilities are approved in Persian (24, 25).
3.3. Procedure
This study was a short-term, randomized, double-blind, placebo-controlled, clinical trial. Participants were randomly assigned to either the “MPH plus piracetam” group or the “MPH plus placebo” group, for six weeks in a 1:1 ratio using a computer-generated code. Parents, patients, and rater were blind to group assignments. The treatment protocol for titrating up of MPH during the study was as follows: starting at 5 mg in morning and mid-day, and weekly increase of 5 mg in each dose until the maximum dose of 40 mg per day during the fourth week. The MPH dose was adjusted for the fifth and the sixth week considering the best dose-treatment response during the first to the fourth week. Methylphenidate was 10 mg round tablets in 20's blisters, and piracetam was 33.3% in 120 mL liquid preparation. The liquid forms of piracetam and placebo (DarouPakhsh Pharmaceutical Company, Tehran, Iran) were similar in color, smell, taste, and viscosity, and their containing bottles were identical in size, shape, weight, and general appearance. The company did not interfere in other parts of the study. The sequentially numbered containers (SNCR) method was used for randomization. One of the researchers allocated the bottles with the code of A or B to the subjects, and another researcher (a senior resident of psychiatry) who was blind to the groups, performed the assessments. As 50 - 70 mg/kg/day is the recommended and well-tolerated dose of piracetam in children (12, 14), a fixed-dose of 60 mg/kg/day in three divided doses was chosen to be administered from the beginning of the first week until the end of the sixth week of the trial for the subjects in the MPH plus piracetam group. Weekly contacts were maintained with subjects and their parents to fill the side effects form and CGI-I at the end of each week; and CPRS-R, CSI-4, and CGAS at the ends of the third and the sixth week. Detailed progress notes were recorded after each contact, as well. Compliance was evaluated by collecting the remaining medication (at the end of the third and the sixth week) and calculating the difference.
3.4. Ethical Considerations
Participation in the research was voluntary, and informed consent and assent were obtained from parents and patients. Both groups received standard medication for ADHD and also piracetam, which was used as add-on treatment and as a well-tolerated medication without serious side effects or drug-drug interaction (12). Subjects' information was protected confidentially, and they could withdraw at any time without reprisal.
3.5. Statistical Analysis
Statistical analysis was done using IBM SPSS Statistics 22. Variables were reported descriptively by mean, standard deviation (SD), and frequency. Comparison of the frequencies of nominal variables was carried out using the chi-square test or Fisher’s exact test. The effectiveness of the treatment was first reported based on paired t-test for outcome measures in each time set. Also, all outcome measures were compared according to the results of repeated measures analysis of variance (ANOVA), considering time as a fixed factor (within-subject variable) and treatment group as between-subject variable. A P-value of less than 0.05 was considered statistically significant.
4. Results
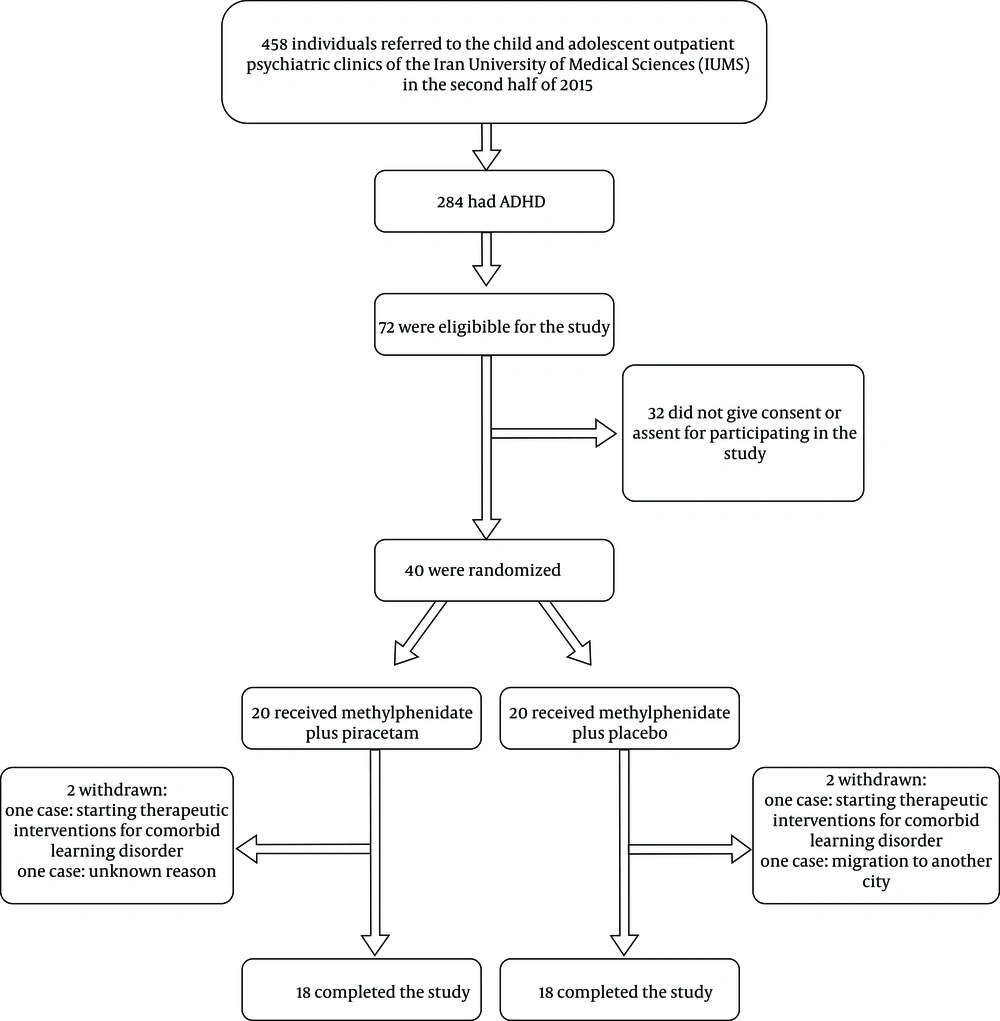
Thirty-six out of the initial 40 subjects who were enrolled in the study completed the whole six-week trial (Figure 1). Four cases left the study before starting the trial: Two cases due to starting interventions for a learning disorder, one case due to immigration, and one case without clear explanation.
The baseline characteristics and comorbidities of subjects are described in Tables 1 and 2. Accordingly, the two groups did not differ significantly in distribution of either baseline demographic or clinical characteristics. Review of systems and physical and neurological examinations in all subjects were within the normal range at baseline and at the ends of the third and the sixth week, and the patients were in the good clinical condition in all visits.
| MPH Plus Piracetam (N = 20) | MPH Plus Placebo (N = 20) | Statistical Comparison | ||
|---|---|---|---|---|
| Test | P-Value | |||
| Respondent (Fr) | ||||
| Mother | 15 | 18 | χ2 = 3.273 | 0.195a |
| Father | 3 | - | ||
| None | 2 | 2 | ||
| Sex (Fr) | ||||
| Male | 14 | 15 | χ2 = 0.125 | 0.723 |
| Female | 6 | 5 | ||
| Educational level (Fr) | ||||
| Preliminary | 16 | 16 | - | - |
| Higher levels | 2 | 2 | ||
| Age, y (mean ± SD) | 9.4 ± 2.0 | 9.6 ± 2.4 | t = 0.227 | 0.822 |
| Height, cm (mean ± SD) | 135.9 ± 11.4 | 137.7 ± 15.6 | t = 0.391 | 0.698 |
| Weight, Kg (mean ± SD) | ||||
| Baseline | 31.3 ± 8.2 | 33.1 ± 12.3 | t = 0.512 | 0.612 |
| After 6 weeks | 31.3 ± 8.1 | 32.8 ± 12.3 | t = 0.450 | 0.656 |
| Type of ADHD | ||||
| Combined | 11 | 12 | χ2 = 0.110 | 0.996 |
| Inattentive | 8 | 7 | ||
| Hyperactive/impulsive | 1 | 1 | ||
Abbreviations: MPH: methylphenidate; ADHD: attention-deficit/hyperactivity disorder; Fr: frequency; SD: standard deviation.
aFisher’s exact test.
Abbreviation: MPH: methylphenidate.
aFisher’s exact test.
4.1. Therapeutic Effect
Tables 3 and 4 show the therapeutic effect of medication packages. According to Tables 3 and 4, the two groups were similar and not statistically different in any of the tests’ scores at baseline. The CPRS and CSI-4 scores showed a significant decrease from baseline to the end of the third and the sixth week in both groups. The CGAS scores increased significantly in both groups during the same time (Tables 3 and 4). Tables 3 and 4 indicate that subjects in the “MPH plus piracetam” group had lower scores in CPRS and CSI-4 at the ends of the third and the sixth week in comparison with the subjects in the “MPH plus placebo” group and the differences between groups were statistically significant. Although CGAS scores changed significantly within each group during the follow-up period, with subjects of the “MPH plus piracetam” group had higher scores; the changes were not significantly different between groups (Tables 3 and 4). Based on the CGI-I scale, 83.3% of subjects in the “MPH plus piracetam” group and 38.8% of subjects in the” MPH plus placebo” group experienced much to very much improvement at the end of the third week, and a significant difference was found between two groups in this regard (P = 0.01, χ = 5.6). At the end of the sixth week, the number of subjects with this level of improvement increased to 88.8% in the “MPH plus piracetam” group and to 66.6% in the “MPH plus placebo” group which was not significantly different (χ2 = 2.57, P = 0.1). This indicates that the therapeutic effect had an earlier onset in the “MPH plus placebo” group.
| Rating Scale & Time | MPH Plus Piracetam | MPH Plus Placebo | t-Test | |
|---|---|---|---|---|
| T | P-Value | |||
| CPRS-R | ||||
| Baseline | 50.9 ± 9.5 | 51.7 ± 7.6 | 0.194 | 0.848 |
| Third week | 21.2 ± 9.9 | 29.6 ± 7.3 | 2.911 | 0.006 |
| Sixth week | 26.8 ± 7.9 | 13.5 ± 10.9 | 4.203 | <0.001 |
| CGAS | ||||
| Baseline | 53.8 ± 8.3 | 52.0 ± 8.0 | 0.658 | 0.515 |
| Third week | 71.2 ± 6.4 | 69.9 ± 6.2 | 0.610 | 0.546 |
| Sixth week | 73.7 ± 6.3 | 70.7 ± 17.5 | 0.673 | 0.506 |
| CSI-4 | ||||
| Baseline | 35.1 ± 7.9 | 37.2 ± 7.0 | 0.856 | 0.398 |
| Third week | 14.8 ± 8.2 | 21.7 ± 5.8 | 2.933 | 0.006 |
| Sixth week | 9.2 ± 7.7 | 18.0 ± 4.9 | 4.096 | <0.001 |
Abbreviations: CGAS, Children's Global Assessment Scale; CPRS-R, Conners' Parents Rating Scale; CSI-4, Child Symptom Inventory-DSM-IV-Version; MPH, methylphenidate; SD, standard deviation.
| Rating Score & Source | F | df | P-Value | ES (Partial η2) | Power |
|---|---|---|---|---|---|
| CPRS-R | |||||
| Time | 232.9 | 1.206 | < 0.001 | 0.873 | 1.000 |
| Time * group | 8.752 | 1.206 | 0.003 | 0.205 | 0.869 |
| CGAS | |||||
| Time | 76.9 | 1.365 | < 0.001 | 0.693 | 1.000 |
| Time * group | 0.123 | 1.365 | 0.805 | 0.004 | 0.065 |
| CSI-4 | |||||
| Time | 188.9 | 1.287 | < 0.001 | 0.847 | 1.000 |
| Time * group | 3.944 | 1.287 | 0.044 | 0.104 | 0.555 |
Abbreviations: CPRS-R: Conner’s parents’ rating scale; CGAS: Children’s Global Assessment Scale; CSI-4: child symptom inventory-DSM-IV-version; ES: effect size.
4.2. Adverse Effects
Table 5 shows the side effects profile. There was no significant difference in side effects between treatment groups. All the mentioned adverse effects were mild, self-limited, not experienced longer than 4 days, and did not interfere with functioning. No subject left the trial because of the adverse effects.
| Disorder | MPH Plus Piracetam | MPH Plus Placebo | Test | Statistical Comparison | |
|---|---|---|---|---|---|
| χ2 | P-Value | ||||
| Abdominal pain | 5 | 6 | Chi-square test | 0.131 | 0.717 |
| Anxiety | 5 | 3 | Chi-square test | 0.643 | 0.423 |
| Decreased appetite | 9 | 11 | Chi-square test | 0.450 | 0.502 |
| Sleep disturbance | 10 | 7 | Chi-square test | 1.003 | 0.317 |
| Drowsiness | 1 | 2 | Fisher’s exact test | 0.364 | 1.000 |
| Dry mouth | 3 | 2 | Fisher’s exact test | 0.232 | 1.000 |
| Headache | 4 | 3 | Chi-square test | 0.177 | 0.674 |
| Irritability | 5 | 6 | Chi-square test | 0.131 | 0.717 |
| Nausea | 3 | 2 | Fisher’s exact test | 0.232 | 1.000 |
| Palpitation | 1 | 2 | Fisher’s exact test | 0.364 | 1.000 |
| Restlessness | 4 | 2 | Fisher’s exact test | 0.800 | 1.000 |
5. Discussion
The pharmaceutical approach is the main treatment modality in ADHD (1), with stimulants known as the first-line treatment (4). However, they cannot be of benefit in a minority of patients (4). Thus, alternative pharmacologic approaches need to be considered seriously. The present study was the first randomized, double-blind, placebo-controlled study, which was performed on children with ADHD, in order to evaluate the effectiveness and safety of piracetam in them. Subjects in both groups demonstrated a significant reduction in ADHD severity and significant improvement in their functioning status during the study. Based on CPRS-R and CSI-4, the decrease in disorder severity had an earlier onset and was more prominent in the piracetam adjunction group. The weight-based dose of piracetam (60 mg/kg/day) used in this trial was within the recommended dose range of piracetam (9-12, 14, 15), and the outcome measures were known as appropriate measures, which are commonly used in treatment studies of ADHD (5, 18-22). In some cases, optimal doses of MPH may be accompanied by problematic side effects, which result in dose reduction. However, reduced doses are sometimes associated with symptoms rebound (4). In such cases, adjuvant therapy may compensate for this problem without exerting additional side effects. Functioning scores based on the CGAS were not affected by the combination of MPH and piracetam in comparison with the control group. Therapeutic effects of the adjuvant experimental medication on the functioning status might be obscured by the prominent effect of MPH in improving the functional domain of the subjects. Also, it is probable that the small sample size and a short period of the present study might not be sufficient to discover the role of piracetam in the improvement of functional status. Subjects in both groups were in good health in all visits. Side effects experienced in both groups were generally mild and did not lead to functional impairment or nonadherence. Similar to our findings, several studies have shown few side effects with piracetam use (8, 10-12).
ADHD results from a developmental lag in CNS functioning and cortical hypo arousal, and some brain areas, which are involved in cognition processing show abnormally low activation rates in ADHD during neurocognitive tasks (1-3). Piracetam is a cerebroactive medication that can improve brain metabolism, information processing, integration and transfer, and global mental and cognitive functions (9-13, 16). Its mechanism of action is based on enhancing mental acts through facilitating the activity of cholinergic, dopaminergic, and noradrenergic systems, maintaining and protecting neuron receptors, and reestablishing impaired neurotransmission (16). These properties of piracetam can be considered as the probable factor for the positive effect of this drug on ADHD.
There are very few researches available in the literature evaluating the possible therapeutic effect of piracetam on ADHD. Zavadenke and Suvorinova (2004) evaluated the therapeutic efficacy of two different doses of piracetam (40 mg/kg/day vs. 70 mg/kg/day) in an open controlled study on children with ADHD. Attention, behavioral characteristics, and motor coordination were improved in both groups, but the response rate was higher in those receiving higher doses of piracetam (14). In another open controlled study on children with ADHD, it was found that monotherapy of piracetam was highly effective in comparison with no pharmacological treatment (15). Our study, which was a double-blind, placebo-controlled randomized trial, consisting larger sample size, revealed almost similar positive therapeutic effects of piracetam as an adjuvant treatment in ADHD. Based on the results of this study, it seems that adjuvant piracetam was effective in decreasing the severity of ADHD symptoms and could reinforce earlier onset and higher therapeutic effects of MPH.
5.1. Limitations
Limitations of the present study, which hinder the expansion of findings were: (1) small sample size; (2) restricted age range; (3) using only referral outpatient cases; (4) short duration (6 weeks) of the follow-up period; and (5) not being able to use Conner’s Teachers Rating Scale (CTRS), because parent’s concern about their child being stigmatized at school.
5.2. Conclusion
The findings of this study provide evidence of positive therapeutic effects and negligible side effect profile of piracetam (60 mg/kg/day) as an adjuvant medication, in the short-term treatment of children with ADHD who are receiving MPH. This medication seems to deserve further comprehensive studies, to explore its efficacy and safety in the treatment of children with ADHD.