1. Background
Memory deficits and age-related memory loss are currently two significant concerns. Memory is permanent storage of learning information while learning is the storage of data acquisition (1). New recommendations and techniques have been developed for memory enhancement and prevention from age-related memory loss. One of these strategies is the use of some medicinal plants with memory enhancing abilities. Kondur is the gum resin of Boswellia serrata that is traditionally used by Iranian pregnant woman for its memory enhancing properties on their fetus (2-4). The beneficial effect of Kondur on the memory deficit of methimazole-induced hypothyroid rats was investigated by the Morris water maze (5). Other studies showed that administration of Kondur aqueous extract during gestational period by mothers improved the learning and memory performance of offspring via an increase in the somal volume of hippocampal neurons in the CA3 region (6, 7). Other different pharmacological such as anti-inflammatory and analgesic effects were also confirmed (8, 9).
Melissa officinalis from the Lamiaceae family is a rich source of natural antioxidant and has therapeutic potency in Alzheimer’s diseases (10), and also in modulation of mood and cognitive performance (11, 12). Badranjboya (Melissa officinalis) is traditionally known for its memory enhancing properties (4). In Iranian Traditional Medicine, there are some prescriptions for combination forms of M. officinalis and Boswellia serrata for memory improvement abilities (13-15).
2. Objectives
As mentioned above, different studies have confirmed the memory-enhancing effects of a single plant extract of Melissa officinalis or Boswellia serrata in animal and clinical trials, thus according to the traditional prescriptions of combined forms of these two extracts (13-15), we evaluated the preventive efficacy of the combined form on memory enhancing and learning potency of scopolamine-treated rats.
3. Materials and Methods
3.1. Plants Material
Flowering aerial parts of Melissa officinalis was collected from a research farm of the medicinal plant research center of Barij (Kashan, Iran) in June 2008. The voucher specimen was identified by Dr Mozaffarian, research institute of Forest and Rangelands, Tehran, Iran, and deposited in the Herbarium of agriculture department, medicinal plants research center of Barij, Kashan Iran, under number 166/1. Boswellia serrata oleo gum resin extract (Batch Number: BS10017) was purchased from Natural Remedies Pvt. Ltd. (Bangalore Karnataka, India).
3.2. Plant Extracts and Phytochemical Analysis
The dried samples of Melissa officinalis were powdered. Next, 0.5 g of fine powdered plant was sonicated twice in 10 mL methanol-H2O (7:3) at room temperature for 30 minutes. After centrifugation, the combined solution was transferred to a 25-mL volumetric flask and was made up to a volume with 70% methanol followed by filtration. For analysis, 10 μL of the filtrate was injected into HPLC (Shimadzu Corporation, Kyoto, Japan); Column: kromasil 100-5 C18 (250 × 4.6 mm), column temperature: 30°C, detector wave length: 330 nm at a flow rate of 1.0 mL/min at 25°C. Two mobile phases included A: 0.1% (v/v) formic acid solution in water, B: acetonitrile with a ratio of 88:12% (first 30 minutes), 80:20% (the next 15 minutes), 70:30% (after 45 minutes). The wavelength of UV detection was 330 nm (16).
For analysis of the B. serrata gum resin extract, 10 mg of the extract was dissolved in 25 mL of 50% ethanol, and then the solution was injected to HPLC. The HPLC instrument conditions were: Column: kromasil 100-5 C18 (250 × 4.6 mm); column temperature: 25°C; detector wave length: 247 nm; solvent rate: 1 mL/minute; washing condition: solvent gradient, running phase was solvent A: methanol, Solvent B: acetonitrile: water (5:95) [pH 2.8 with orthophosphoric acid]; washing program: time (0, 90) [A (90%): B (10%)]. The amount of total boswellic acid, 11-keto-B-boswellic acid, 3- acetyl-11 keto β-Boswellic acid were determined by a calibration curve (17).
3.3. Animal Model of Memory
Adult male rats of Wistar strain weighing 200-220 g were purchased from Kashan University of Medical Sciences (Kashan, Iran). They had free access to food and water ad libitum. The experimental subjects were kept in a single holding room and housed at a constant temperature of 21 ± 2 0 c, humidity of 55 ± 5% and under 12-hour light/dark cycle. All experiments were in accordance with the UK Animals Scientific Procedures Act 1986 (86/609/EEC). The rats were randomly assigned to four experimental groups: groups 1 and 2: B. serrata and M. officinalis combination (200 mg or 130.6 + 69.4 mg Kg-1) (n = 10), B. serrata and M. officinalis combination (400 mg or 261.2+138.8 mg Kg-1) (n = 10), respectively, group 3: scopolamine (n = 10) and group 4: normal group (n = 10). The control group was the positive control and included animals with normal memory. Groups 1 and 2 were pretreated with combination of plant extracts (200 and 400 mg Kg-1 orally) daily for four successive weeks at 4 pm, while other groups (3 and 4) received distilled water orally daily for four weeks. After this time (four weeks), 30 minutes before starting the experiment, groups 1 and 3 were intraperitoneally injected by the same dose of scopolamine (0.1 mg Kg1). After 30 minutes of all treatments at every time interval, rats of all groups were subjected to Morris water maze task for four days (two trials/day at 7 pm).
Spatial learning performance was assessed in a tank made of galvanized metal, 150 cm diameter, 70 cm depth and filled with water at 22°C up to 20 cm below the rim. The pool was divided to four equal quadrants named northeast, southeast, southwest and northwest. A 10-cm circular platform was submerged 1.5 cm below the water surface and located in the center of one quadrant in a fixed position throughout the experiment. The testing room contained a number of extra-maze visual cues, visible to the experimental subjects. A TV camera and computerized tracking system were utilized to monitor and preserve the animal navigation in the water maze. Behavioral variables were measured with the help of customized software (Radiab 7, IR Iran). For behavioral testing, each trial was started by releasing an animal into the water maze, immediately facing the perimeter, at one of four starting points. During the training phase of the experiment, the animals were allowed to swim for a maximal time of 60 seconds to find the hidden platform. If the subject failed to find the platform within 60 seconds, it was guided to the platform where it remained for 20 seconds and had a 15-min inter-trial interval. Then, the rat was immediately replaced in the pool. The starting position was changed in each test. Following the completion of the trial, the rats were dried and returned to their home cage. This stage of the experiment took four consecutive days, each subject having four training trials per day. The number of trials in which the animals could locate the hidden platform before elapsing the maximum time of 60 seconds, the delay and swim path to locate the platform, were measured as indices of learning of the spatial task.
For the spatial memory retrieval test, on day five, the animals were introduced to a probe test to assess the animal’s retrieval performance of spatial memory. Therefore, the platform was taken out from the maze and the animals were liberated from the quadrant opposite to where the platform was and allowed to swim freely for 60 seconds. Spatial retention in probe trials was assessed by the percentage of total time expended in the platform quadrant during the preparation phase (18, 19).
3.4. Statistical Analysis
The total time elapsed to locate the platform (in seconds) and the distance swam by the animal until it found the platform (in centimeters) was taken as indicators of learning in the training phase of the experiments. The data pooled from the training were normalized and presented as a mean ± standard error of the mean (SEM) and analyzed by a repeated measurement two way analysis of variance (ANOVA) followed by an LSD post test.
4. Results
Analysis of M. officinalis extract showed the presence of rosmarinic acid (8.5% w/w). Total boswellic acids, 11-keto-β-boswellic acid and acetyl-11-keto- β-boswellic acid were 70%, 4.67% and 1.85% w/w in B. serrata extract.
The pretreatment of rats with 200 and 400 mg/kg of B. serrata extract on memory of scopolamine treated rats had an effect on memory of scopolamine-treated rats lower than the combined form of M. officinalis and B. serrata extract equal to 200 and 400 mg/kg (unpublished data).
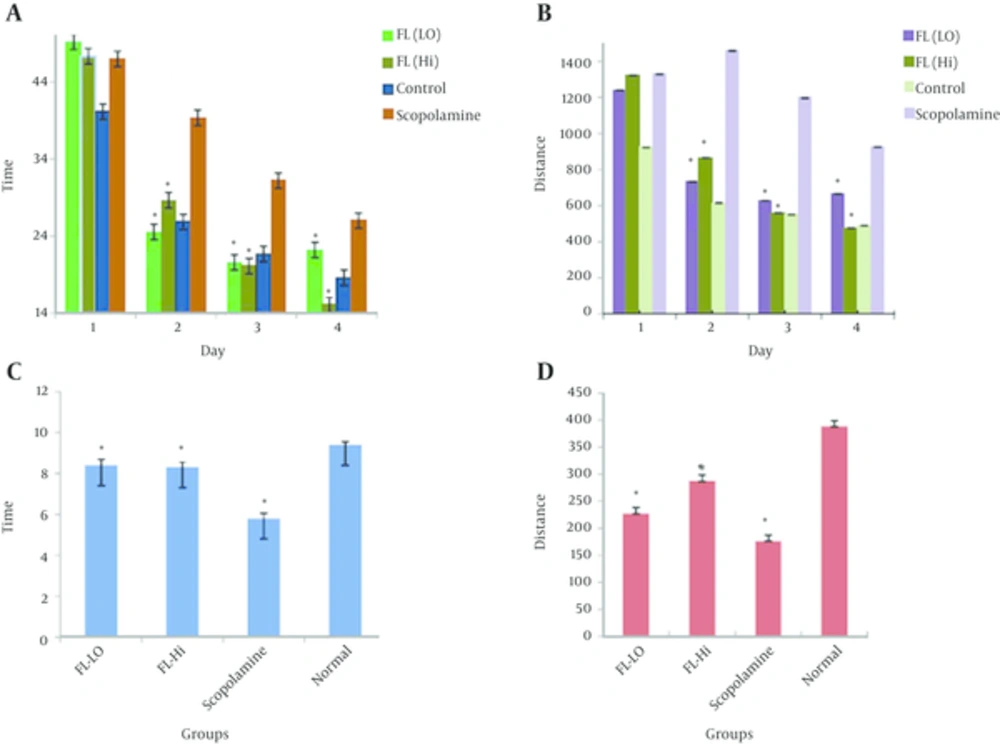
In this work, we estimated the means of Distance (D), and time expended in the target quadrant (T), in all groups, and the resolutions were recorded as mean ± SEM (Figure 1A, 1B, Table 1).
| Groups | Day 1 | Day 2 | Day 3 | Day 4 | ||||
|---|---|---|---|---|---|---|---|---|
| T | D | T | D | T | D | T | D | |
| FL (200 mg) | 49 ± 7.7 | 1240 ± 196 | 25 ± 3.8 | 733.2 ± 116 | 21 ± 3.2 | 628.5 ± 99.4 | 22 ± 3.5 | 665 ± 105.1 |
| FL (400 mg) | 47 ± 7.8 | 1323 ± 220 | 29 ± 4.7 | 865 ± 144.2 | 20 ± 3.3 | 561 ± 93.5 | 15 ± 2.5 | 476 ± 79.3 |
| Scopolamine | 47 ± 7.4 | 1328 ± 210 | 39 ± 6.2 | 1458 ± 230.5 | 31.2 ± 4.9 | 1197 ± 189.3 | 26 ± 4.1 | 925 ± 146 |
| Control | 40 ± 6.3 | 923 ± 146 | 24 ± 4.1 | 616 ± 97.4 | 22 ± 3.4 | 550 ± 89.6 | 19 ± 2.9 | 490 ± 77.4 |
Abbreviations: FL, Boswellia serrate, Melissa officinalis; T, time; D, distance.
After four days, the time spent in the target quadrant decreased in all groups, this means with spatial keys, all animals learned the location of the hidden program. There was a significant difference between the scopolamine and control groups for time spent (P = 0.01). There was no significant difference between group 1 and 2 (200 and 400 mg/kg) and control groups (P = 0.09), while groups 1 and 2 had a statistically difference with the scopolamine group (P = 0.000). The results of groups 1 and 2 relieved that the deterioration is due to scopolamine administration and co-administration of plant extracts improved the storage ability of rats. A significant difference was observed between groups 1, 2 and 4, and the positive control group in path length to goal (group 3) (P = 0.01).
For distance, there was a significant difference between scopolamine with impaired memory and control group with normal memory (P = 0.03). Groups 1 and 2 (200 and 400 mg/kg) had a significant difference with the scopolamine group (P = 0.015) and did not have any difference with the control group (P = 0.09).
The spatial memory evaluation by the probe for assessing the time showed a significant difference between other groups (including treated and normal groups) and the scopolamine group (P = 0.03), yet no significant difference was observed between treated groups and the normal group (P = 0.09). The distance probing showed a difference between normal and scopolamine groups, while a difference between treated groups and scopolamine group was also observed. There was also a significant difference between treated groups and normal group in distance probing (P = 0.015). Therefore, in regards to time, treating the rats by a combination of extracts can correct the impairment of memory by scopolamine and this effect was the same as the normal group. In regards to the distance, the co-administration of plant extracts could prevent the impairment of scopolamine, but this efficacy was not the same as the normal group (Figure 1C and 1D, Table 2).
| Means ± SEM | ||
|---|---|---|
| Time | Distance | |
| FL (200 mg) | 8.4±0.29 | 277.4±8.25 |
| FL (400 mg) | 8.3±0.28 | 287.3±10.8 |
| Scopolamine | 5.8±0.25 | 176.6±7.37 |
| Control | 9.4±0.14 | 388.4±8.63 |
Abbreviations: FL= Boswellia serrata, Melissa officinalis
5. Discussion
In this study, we evaluated the preventive efficacy of co-administration of B. serrata and M. officinalis against impaired memory in scopolamine-treated rats. Scopolamine is an alkaloid drug that is medicinally used for treatment (20). The mortal role of scopolamine in the cholinergic system for learning and memory is shown in animal models (21). Therefore, we prospected that scopolamine-treated rats showed higher distance and time in the target quadrant as compared with rats with normal memory. In this study, the preventive efficacy of M. officinalis and B. serrata in learning, and memory enhancement in cognitive impairment related to scopolamine were shown. Scopolamine acts as an antagonist acetylcholine receptor with amnestic properties and inhibits the binding of acetylcholine to muscarinic receptors; therefore, blocks the nerve impulses that are mediated by acetylcholine and also enhances Acetylcholinesterase (AchE) activity (22). It is reported that acetylcholine, as a major neurotransmitter in cholinergic neurons, has essential activity in learning and memory neurons (23). Blocking of muscarinic receptors is an essential element for controlling the central nervous system that induces cognitive deficits (24). Memory impairment related to scopolamine is also associated with oxidative stress in the brain (25). Therefore, antioxidant and oxidative free radical scavenging components have an important role in reducing oxidative stress. The antioxidant activity of B. serrata (45 or 90 mg/kg) in the cerebrovascular system has been revealed (26). The effects of B. serrata were evaluated in AlCl3-treated animals with Alzheimer’s diseases by measuring the level of acetylcholine and acetylcholine esterase and behavior stress test. The results of the study exhibited that B. serrata infusion extract showed significant improvement in animals with Alzheimer’s diseases and increase the brain acetylcholine levels, and also significantly decrease brain acetylcholine esterase levels, dose dependently. Amyloid plaques have been shown to disappear by B. serrata extract. Therefore, the therapeutic and protective effects of B. serrata could be attributed to its antioxidant activity (27).
Boswellic acids, as the major components of B. serrata are reported to have anti acetylcholine esterase activity (28).
Inflammation also has an important role in pathogenesis of Alzheimer’s and memory diseases. Brain inflammation impaired spatial learning and memory function (29). Memory deficits may be correlated with inflammation in the brain via expression of inflammatory cytokines and chemokines. The anti-inflammatory effect of B. serrata is also attributed to suppression of leukotrienes via inhibition of 5-lipoxygenase (5-LO). Keto boswellic acids are non-competitive blockers of 5-LO. The leukocyte population and infiltration of leukocytes decrease by boswellic acids (30). Boswellic acids interfere with Prostaglandin 2 (PGE2) formations and reduce edema in patients with malignant tumor (31, 32). Therefore, blocking of 5-LO enzyme may have some functional role in blocking of cognitive decline, neurofibrillary tangles and amyloid deposition (33). Also, corticosteroids via 5-LO damage to synapse impair memory and learning. The 5-LO enzyme is over expressed in Alzheimer disease and knockout of 5-LO gene decreases the amyloid β levels (33). Reduction in 5-LO decreases the 5-hydroxyeicosatetraenoic acid (5-HETE), leukotriene B4 (LTB-4) and other inflammatory cytokines (9). Boswellia serrata is known as an orphan drug for treatment of peri-tumor brain edema (31).
It is reported that M. officinalis ethanolic extract improves memory and learning ability in normal and scopolamine treated animals and this activity is not dose dependent (23). Melissa officinalis extract and, especially, the main components of M. officinalis extract, rosmarinic acid and chlorogenic acid have anti-cholinesterase (22, 23, 34) and neuroprotective activity against amyloid β-induced toxicity (35). Therefore, different mechanisms of 5-LO and inflammation blocking, and reduction of free radicals and acetylcholine esterase is responsible for memory enhancement and learning properties of co-administration of two plant extracts.
Our results showed that the combination of two plant extracts could prevent memory loss as a result of scopolamine treatment. Therefore it is not possible to conclude that the combination of B. serrata and M. officinalis extracts has the potency for memory enhancement and learning abilities.
5.1. Conclusion
In conclusion, the improvement in status of memory after administration of M. officinalis and B. serrata extracts may be related to antioxidant, anti-inflammatory or anti-acetylcholine esterase effects of extracts on damaged brain cells or may be a result of protective effects of extracts against the toxic effects of scopolamine on healthy brain cells. More researches are required to investigate their mechanisms of action. It is important to evaluate the potency of combined form of extract in clinical trials. Three groups of people should be the subjects of clinical trials including pregnant women, school age children and elderly. Before starting clinical trials, it is important to evaluate the toxicological aspects of these combinations in animal models.