1. Background
Memory is the ability to retain and recall information during thinking (1). Memory is an important key for performing diverse cognitive tasks such as reading comprehension and problem solving. Cognitive abilities are essential for educational achievements, social, and vocational skills training (2). Memory performance is strictly contributed to cognitive functions, so it is vital to investigate diverse strategies to enhance memory performance. One of the most promising strategies to improve memory performance is neurofeedback training (3), a type of electroencephalogram biofeedback. Biofeedback is defined as measuring a quantifiable bodily function, for example, muscle tension, pulse rate or brain electrical activity, and supplying real time information about bodily functions. Neurofeedback provides auditory and/or visual information about brain waves. Desirable brain activity is rewarded based on this information. This process is based on the operant conditioning principle in which the individuals learn to regulate their brain frequencies through positive reinforcement during training sessions (3-5). Neurofeedback protocols define desired frequency patterns that need to be practiced (6).
Cognitive processes are closely correlated with brain waves; brain frequencies have a different role in particular cognitive processes including memory (7). Studies investigated the effects of neurofeedback on cognitive performance of healthy volunteers (2, 8). However, Egner et al. has questioned the effectiveness of neurofeedback training (4) in improving memory performance and treating a variety of psychiatric disorders (5).
Alpha waves are neural oscillations in the frequency range of 7 to 12 Hz. It is assumed that they improve memory performance through inhibition of irrelevant information (9). Upper or high alpha (9.5 - 12 Hz) and lower alpha (7 - 9.5Hz) frequency are related to cortical storage and thalamocortical neural activity (10). Several studies have suggested that increasing the upper alpha frequency is related to enhancement in cognitive performance and working memory (11-14), and desynchronizing upper alpha frequency correlates with semantic memory performance (2). This decrease in synchrony strongly correlates with the formation and retrieval of long-term memories (15). Studies have suggested that the effects of lower alpha on cognitive performance may be contrary to upper alpha (2, 12). Thus, suppression of lower alpha at the same time with increasing upper alpha may enhance cognitive performance (12).
Nonetheless, alpha changes have not indicated significant effects on learning and memory tasks after feedback training studies (16, 17). These findings point towards the need for more research to investigate the effects of high alpha potentiation and low alpha suppression (or low alpha/high alpha ratio suppression) in memory performance (12).
Beta frequency is between 12 to 20 Hz, with low amplitudes contributed to memory (15), waking consciousness, and a state of alertness (7). Gamma frequency ranges from 30 to 44 Hz (18). It seems that increases in these frequency ranges affect memory formation via shaping synaptic plasticity and coordinating the reactivation of memories (15). While beta and gamma neurofeedback training have not improved cognitive performance in older adults (19), another study confirmed that working memory can be improved by theta activity in normal aging adults (3). Other studies have indicated that increasing beta frequency and decreasing high theta and low alpha frequencies improve the state of attention (6). These results suggest that the role of beta frequency in improving memory performance should be more explored.
Theta frequency ranges from 4 to 8 Hz in the frontal midline region. Studies have shown that theta frequency is related to working memory, attention, and memory consolidation (3, 20). Theta frequency neurofeedback training can enhance memory consolidation (7), memory updating, and mental set shifting (21), encoding new information into episodic memory or working memory (2), recognition memory (22), and memory performance (10, 23, 24).
Finally, it has been inferred from the studies conducted in neurofeedback and memory performance that the association between brain frequencies and memory performance needs to be explored further. In addition, the generalizability of previous studies is limited because of methodological issues, eg, lack of control groups (12, 17), and open rather than blind design of studies. While theta/beta ratio neurofeedback has already been investigated (19), no study has been conducted on low alpha/high alpha ratio suppression training. The additional suppression of lower alpha may simultaneously improve cognitive performance (12).
2. Objective
This was a randomized, double-blind, sham-controlled study that investigated the effects of 2 neurofeedback protocols (beta up-training/theta down-training and low alpha/ high alpha ratio suppression) on memory performance.
3. Materials and Methods
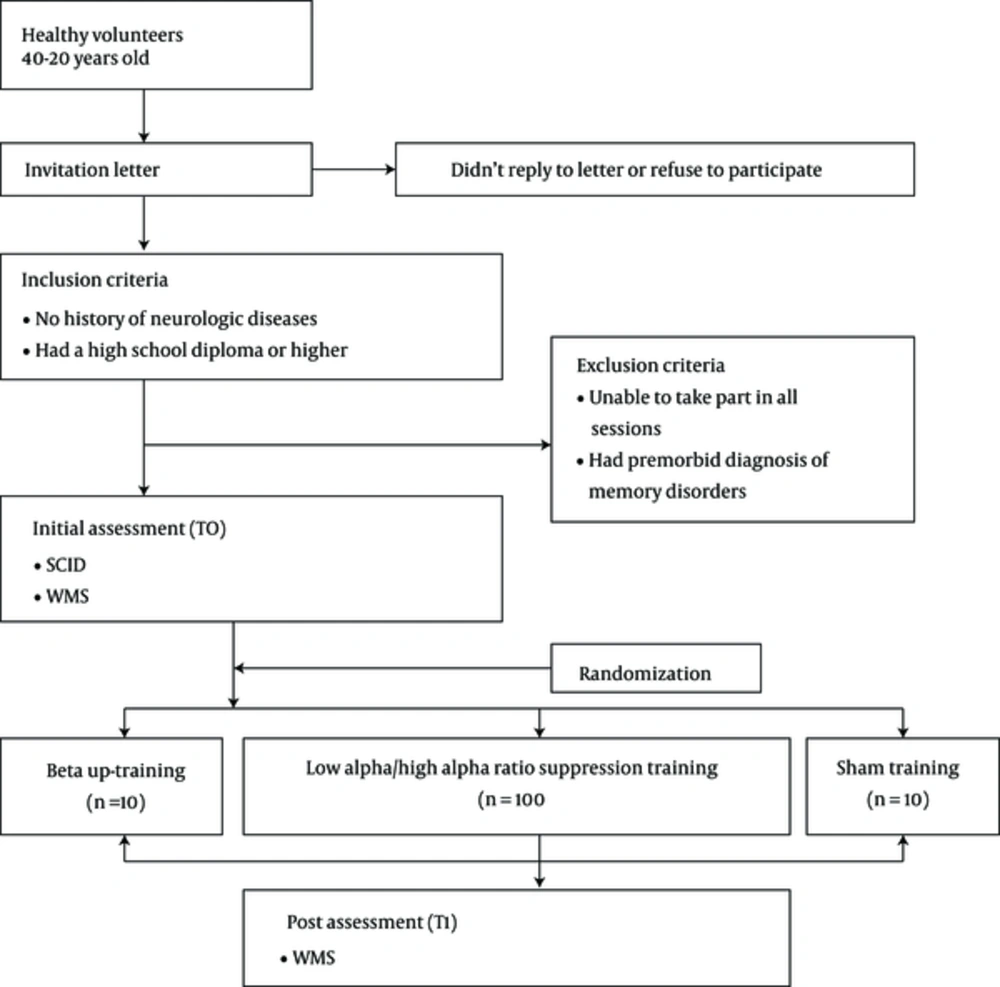
This was a randomized, double- blind, sham-controlled, trial performed at Zare hospital in Sari, Mazandaran, Iran, during 2014 and 2015. Healthy adult employees working at Zare hospital were invited to participate in the present study. They were randomly selected based on employing number using random number table. After accepting the invitation, the participants underwent the screening process. Inclusion criteria were as follows: (1) age = 20 to 40 years, and (2) educational level = diploma and upper. Exclusion criteria were as follow: (1) history or current diagnosis of any psychiatric disorder (eg, mood disorders, anxiety disorders, etc.); (2) known neurological diseases (eg, seizure disorder, head trauma, stroke, amnestic disorder, mild cognitive impairment, dementia, etc.); (3) history or current use of drugs and substances (eg, benzodiazepines, barbiturate, methamphetamine, ethanol, etc.); (4) night/shift work employment; (5) pregnancy and breastfeeding; and (6) not being able to fully take part in all sessions.
Using the Structured Clinical Interview (SCID) for DSM-IV-TR to rule out psychiatric diagnoses, a psychiatrist interviewed all participants.
The structured clinical interview for DSM-IV Axis I Disorders (SCID-I) (25) is a semi-structured interview for making the major DSM-IV Axis I diagnoses. The SCID-II is a semi-structured interview for making DSM-IV Axis II: Personality Disorder diagnoses.
Written informed consent was obtained from all participants after providing them with adequate information about the study. The study was approved by the research ethics committee of Mazandaran University of Medical Sciences. The Wechsler memory scale (WMS-R) was administered to all participants directly before (T0) and after (T1) the training period to examine memory performance. WMS-R, comprising auditory memory, visual memory, visual working memory, immediate memory, and delayed memory was conducted by a trained psychologist in a 90-minute session. The psychologist was not aware of participants’ assignment to their groups throughout the whole study. The WMS-R has been standardized for Iranian population (26). It is one of the most commonly used scales for assessing memory performance (27). This version is remarkable due to the increased normative information in the revised scale (28). Reliability coefficients were satisfactory and ranged from 0.28 to 0.98 for the subtests, and validity was investigated, in which patients with memory impairment scored lower than healthy individuals (26).
Thirty participants were eligible to join the study. Participants were randomly assigned into 3 groups by block randomization: (A) beta up-training and theta down-training, (B) low alpha/high alpha ratio suppression, and (C) a sham control group that did not receive neurofeedback training (Figure 1). Each person received 10 sessions of neurofeedback training every other day of the week. Each session lasted 30 minutes and comprised two 7-minute training blocks separated by a 6- minute break. The participants sat quietly with eyes closed and did not leave the training room during the breaks. They were blind to neurofeedback protocols.
Another member of the research team (a trained psychologist) was responsible for providing neurofeedback training and had no role in data collection and analysis. The ProComp2 InfinitiTM encoder was used for real time computerized biofeedback and data collection. Training sessions were held in the clinic using the thought technology ProComp2 infiniti (SA7500). The electrode for recording EEG was placed at a central location, FCz (midway between Cz and Fz) during all 3 protocols. Participants were reinforced with an auditory or visual stimulus (eg, bell tone, obtaining high scores, and winning the game, and solving puzzles) to keep brain waves within a desired threshold except that sham module produced random reinforcements during training. Indeed, neurofeedback therapy is like a computer game, but has a fundamental difference. It is completely hands-free and the patients should guide the energy and ability of their brain game.
The statistical package for social sciences (SPSS Inc., Version 20, Chicago, IL, USA) was used for data analysis. To analyze the changes in memory performance, analysis of covariance (ANCOVA) was conducted on the difference in the mean scores of Wechsler memory scale among the 3 groups before and after the intervention (Figure 2). Analysis of variance (ANOVA) and Fisher’s exact test were conducted to assess statistical significance among groups in age, gender, marital status, and educational level. Statistical significance was considered at (P < 0.05).
4. Results
The mean age of the participants in groups A and B was 31.7 years (SD = 6.65) and 31.7 years (SD = 6.40) for the experimental group, and 34.2 years (SD = 5.70) for the control group. The result of ANOVA (P = 0/593) revealed no statically significant difference between the 3 groups in age.
Moreover, distribution of gender, education, and marital status was normal. The results of Fisher test showed no statically significant difference between the 3 groups in gender (0/879), education (1), and marital status (0.51). Participants’ demographic features are presented in Table 1.
aA, Beta Up-Training and Theta Down-Training; B, Low Alpha/High Alpha Ratio Suppression Training; C, Sham Training.
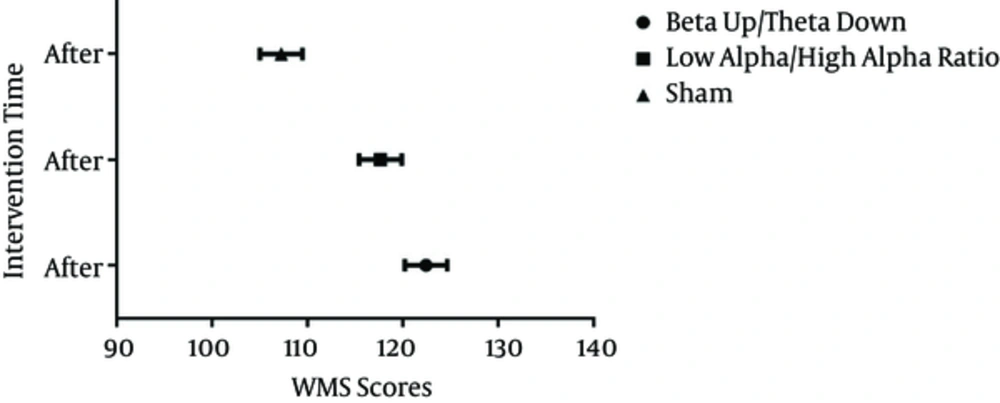
The Shapiro-Wilk test was used to examine the normality of distribution for WMS-R scores (P > 0.05). ANCOVA was statistically significant (Table 2). There was an overall statistically significant difference in postintervention memory performance among the different frequency trainings after adjusting the means once their means had been adjusted for preintervention memory performance (Table 2). Table 3 demonstrates how the covariate has adjusted the original post group means (Table 3). The results of analysis of covariance (ANCOVA) for each variable revealed that in the posttest, the mean score of memory performance in group A was P = 0.015, and in group B was P = 0.05, meaning that the 2 protocols of neurofeedback were effective in improving memory performance in groups.
| Parameter | B | Std | T | Sig | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Error | Lower Bound | Upper Bound | ||||
| Intercept | 25.281 | 8.309 | 3.043 | 0.005 | 8.202 | 42.361 |
| Y-PRE | 0.772 | 0.077 | 9.994 | 0.000 | 0.614 | 0.931 |
| Beta up/theta down | 15.206 | 3.148 | 4.830 | 0.000 | 8.734 | 21.677 |
| Low alpha/high alpha ratio | 10.424 | 3.159 | 3.300 | 0.003 | 3.932 | 16.917 |
| Sham | 0 | |||||
| Intervention Group | Mean | Std. Error | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Beta up/theta down | 122.462 | 2.220 | 117.898 | 127.026 |
| Low alpha/high alpha ratio | 117.681 | 2.225 | 113.107 | 122.255 |
| Sham | 107.257 | 2.228 | 102.676 | 111.837 |
5. Discussion
Neurofeedback is a useful, noninvasive, and inexpensive treatment for several psychiatric disorders, with recent research investigating applications for enhancement of cognitive ability in nonclinical populations (29-31). In our study, both neurofeedback training groups showed significant improvement in memory performance of healthy adults. Few studies have focused on lower beta up-training to improve cognitive performance (15, 32). On the contrary, theta frequency has considerably been implicated in cognitive functions, especially memory performance (1, 2, 7, 20-23). Similar to our study protocol, Rasey et al. showed that enhancing beta activity and inhibiting high theta and low alpha activity may associate with improved attention. However, they used the Wechsler adult intelligence scale revised (WAIS-R) to obtain intelligence quotient to identify IQ scores, but they did not report memory performance trends among participants (6); this finding has also been supported by Egner and Gruzelier who found that enhancing low beta and simultaneously inhibiting theta may lead to improved attention among healthy adults (33). Haddadi et al. found that beta frequency up-training and theta frequency down-training in T3 and F3 may enhance learning and memory among patients with cognitive impairment (34). These results may be due to the general attention-increasing and arousal-enhancing effect of beta training (32). It seems that increases in these frequency ranges, affect memory formation via shaping synaptic plasticity and coordinating the reactivation of memories (15).
The findings of our study could put in new information into a body of existing knowledge about neurofeedback implication in young adults whom may benefit from beta up-training and theta down-training neurofeedback program to improve their memory performance.
Anther protocol indicated that low alpha/ high alpha ratio in suppression training program has also improved memory performance among healthy young adults. The underlying mechanism for memory improvement may be related to inhibition of irrelevant information (9) in cortical storage and thalamocortical neural activity (10). Increasing high (upper) alpha frequency suppresses distracting stimuli and inhibits unnecessary and conflicting processes, thus, facilitating memory and task performance (35). However, several studies supported that upper alpha frequency training has enhanced memory performance in diverse population (10-14).
Bauer concluded that changes in alpha frequency had no significant difference for the learning process (16). Vernon had discussed that more research should be done to investigate the role of alpha frequency in cognitive process (24). Thus, consistent with Zoefel et al. who indicated that upper alpha training improves cognitive performance (12), our study showed that minimizing the lower/upper alpha ratio has a significant impact on memory performance. Vernon also reminded that significant improvement in cognitive process may stem from splitting up the alpha frequency training into lower and upper alpha band (24); our finding confirmed this hypothesis that low alpha/high alpha ratio suppression training may improve memory performance.
A limitation of the current study was its convenience sampling method. Further, We only performed the neurofeedback training on healthy young adults working at Zare hospital, in Sari, North of Iran, so the sample was not representative of the entire population. Thus, there is the opportunity for bias to cloud the results of the study.
Many factors may influence the success of neurofeedback procedures such as age of the trainees, their personal traits and beliefs about neurofeedback training, training susceptibility of participants, trainer behavior, feedback modality (visual, auditory, combined), training intensity, choice of EEG used for the feedback signal, and the number and positions of electrodes (36-38). However, we could not consider all of these factors in our study design and analysis.
Because learned helplessness effect might have happened among the participants in the sham group, this study could be replicated with another control group, in which no intervention would be implemented. Learned helplessness may happen, while participants learn that what they did had nothing to do with the outcome, so it may bring about passive behavior as well as low scores (7). This effect may violate the results. A significant difference was assumed between the treatment and the sham group, which may stem from the learned helplessness. Whereas the Wechsler Memory Scale-Revised as one of the most common psychological scales has showed acceptable reliability and validity among healthy population, other cognitive domains were not assessed in our study (39).
This paper has shown that beta up-training and theta down-training as well as low alpha/high alpha ratio suppression training significantly improved memory performance in healthy adults. This study mainly extends the work of Escolano et al. (40) and Zoefel et al. (12) by assessing the frequency during neurofeedback sessions and evaluating memory performance scores before and after training sessions to explore the effects of components of alpha frequencies on memory performance. In addition, beta up-training neurofeedback protocol has been conducted on healthy adults to investigate the role of upper beta in memory improvement. It is recommended to investigate the effect of particular neurofeedback protocols, on a wider age range, on patients with cognitive impairment and for a longer period to assess and follow- up the long-term effects on memory and other cognitive functions. Finally, particular neurofeedback training sessions were assumed to improve memory performance compared to the control group. Future studies should address the specificity of the neurofeedback training effects in diverse populations.