1. Background
Motor imagery (MI) as a complex cognitive function is defined by the ability to mentally perform movement without overt movement execution.
Parson’s hand rotation task (HRT) is usually used to investigate the MI ability. In this task, subjects should detect the laterality of right/left-hand pictures which are rotated in different angles (1, 2). Larger the rotation angle of the pictures is, more the error rate will be. The response time is also recorded to indicate the engagement of subjects in a cognitive function of mental rotation.
Among cognitive processes involving in mental rotation, working memory is crucial. Particularly, both mental rotation and spatial working memory (SWM) imply the processes of encoding, maintaining, and transforming spatial information. Indeed, information about a mental rotation execution has to be processed from long-term memory to working memory. The image not only should be transformed within working memory (e.g., by rotating a visual image), but also has to be maintained. Moreover, the image should be inspected with the aim of detecting detail to deliver a response (3, 4).
Patients with schizophrenia demonstrate impairments in working memory across a wide range of experimental tasks (5). Intact performance on the tasks requires sufficient encoding and maintenance of information over a short period of time. In particular, impaired spatial working memory function is considered as an effective endophenotypic marker for schizophrenia (6).
Studies have shown impaired mental imagery ability in patients with schizophrenia (7-10). More reaction times and error rates were reported in patients with schizophrenia by de Vignemont and colleagues on HRT, while slow and inaccurate responses increased with larger angles of rotation in patients as did in controls. They suggested that the attention and working memory deficits lead to less accurate responses in patients, but they did not investigate these relationships in their study (7).
While several lines of evidence suggest impaired mental rotation in schizophrenia, a few studies have explored the underlying cognitive mechanisms in the patients. To the best of our knowledge, the relationship between the MI abnormalities and SWM deficits in patients with schizophrenia has not been studied before, which was the main goal of this study. To reach this goal, we examined performances of a group of patients with schizophrenia on HRT and Dot test of visuospatial working memory.
2. Objectives
To the best of our knowledge, the relationship between the MI abnormalities and SWM deficits in patients with schizophrenia has not been studied before, which was the main goal of this study. To reach this goal, we examined performances of a group of patients with schizophrenia on HRT and Dot test of visuospatial working memory.
3. Materials and Methods
3.1. Patients
The study sample included 18 patients (four males) with schizophrenia who were selected from a psychiatric hospital (Golestan Salamat in Kerman) from 2012 to 2013. All of them diagnosed by an expert psychiatrist using DSM-IV Criteria based on structured clinical interviews and their medical records. They were evaluated using the scale for assessment of positive symptoms (SAPS) and the scale for assessment of negative symptoms (SANS) (11, 12). During the test, patients used antipsychotic drugs and were clinically stable. The mean chlorpromazine equivalent dose was 318.5 mg (13). Subjects with comorbid diagnoses of schizoaffective disorder, mood disorders, substance abuse disorders, or history of head trauma and neurological disease were excluded. The control group included 18 healthy subjects (four males) screened for a personal or family history of the psychotic disease. The two groups were matched for age, gender, and education. All participants were right hand dominant (Table 1) and had normal or corrected-to-normal vision. A written informed consent was obtained from the subjects. The study was approved by the Ethics Committee of Kerman University of Medical Sciences and the ethical code number was 93 - 22.
| Patients | Controls | |
|---|---|---|
| Age, y | 32.36 ± 7.53 | 31.11 ± 7.37 |
| Sex, M/F | 4/14 | 4/14 |
| Education, y | 12 ± 2.6 | 12 ± 2.48 |
| Handednessa | 94.15 ± 13.3 | 94.08 ± 10.69 |
| Disease duration, y | 10 ± 6.38 | |
| SAPS | 19.4 ± 14.5 | |
| SANS | 38.2 ± 15 | |
| Chlorpromazine equivalent dose | 318.5 ± 165.2 |
aHandedness = A laterality quotient (≥ 50 right handed) assessed with the Edinburgh handedness inventory.
3.2. Procedure
3.2.1. Hand Rotation Task
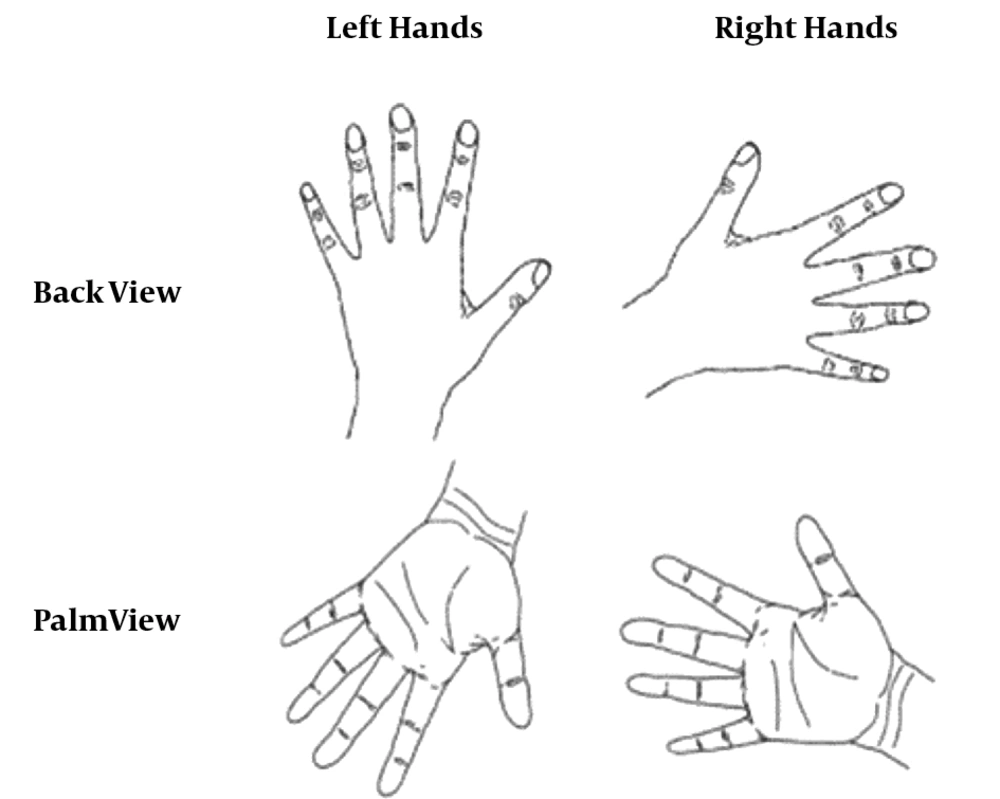
To examine motor imagery, the Parson’s classical HRT is often used (2). During this task, subjects need to detect the laterality of right/left-hand pictures that are rotated in different orientations. The subjects were seated in a quiet testing room. The pictures of right/left hand in the palm and back views (Figure 1) were demonstrated in a random order. The pictures had six different rotation angles (0°, 60°, 120°, 180°, 240°, and 300°). Totally, 360 trials resulted from the 15-time repetition of each angle (6 × 2 × 2 × 15). The subjects were asked to detect the laterality of pictures by pressing the right or left button as accurately and quickly as possible. Reaction times and percentage of correct responses (accuracy rate) were recorded by a key press. Trials in which reaction times were three or more standard deviations above the mean were eliminated prior to the analysis.
3.2.2. Dot Test of visuospatial Working Memory
This test is used to measure visuospatial working memory (14), which as a simple task is less vulnerable to performance problems such as a motivation and uncooperativeness in schizophrenic patients. The participants sat in front of a table and they were given a flat-tip marker. The test includes immediate and delayed trials. On 14 immediate trials, a filled black dot with a 0.5-cm diameter (as a stimulus) was presented on a piece of standard white paper located in a landscape orientation. In each trial, the dot was semi-randomly displayed three or four times in each quadrant. The blank white paper as a response page was placed below the stimulus page and the subjects were instructed to mark the location of stimulus in the same place onto the response page. The distance between the outer edge of the black dot and the center of the subjects’ mark was measured to calculate the immediate performance. Then, the immediate 14 trials were averaged together.
During the delayed visuospatial performance, the subjects were instructed that there would be a delay (10-, 20-, or 30- second) before marking on the response page. The black dot was presented for five seconds and then the stimulus paper was removed. The participants were asked to recall the location of stimulus during the delay period. The delay trials included three parts: Trials 15 - 24, 25 - 34, and 35 - 44, which were performed with 10-, 20-, or 30- second delay in a random order. During the delay period, the subjects were provided with the different lists of words, which were read aloud to prevent verbal remember of stimulus location. Distances in delay performances were also measured similar to immediate trials. The performances over 10-, 20-, and 30- second trials were averaged to calculate the 10-, 20-, and 30- second delay visuospatial performances, respectively. The WM deficit was obtained by subtracting the immediate performance from the total average of the 10-, 20-, and 30- second delay performances.
Then, the data were analyzed using repeated measures ANOVA by SPSS 13.00 statistical software considering a P value of 0.05.
4. Results
All of the participants (22 subjects in the patient group and 22 subjects in the control group) completed all stages of the study. Their demographic and clinical characteristics are shown in Table 1. There were no significant differences in age, sex, and education between the groups (Table 1)
4.1. Hand Rotation Task
The mean scores and SD of the response reaction times and the accuracy of the subjects are shown in Table 2.
| Patients | Controls | P Value | |
|---|---|---|---|
| Reaction time (total), ms | 1913.5 ± 406.5 | 1496.9 ± 246.33 | < 0.05 |
| Small angles | 1756.21 ± 365.32 | 1371 ± 233 | < 0.05 |
| Large angles | 2088.95 ± 465.1 | 1679.2 ± 247.27 | < 0.05 |
| Accuracy (total), % | 82.45 ± 13.5 | 91.12 ± 7.11 | < 0.05 |
| Small angles | 85.81 ± 12.1 | 92.22 ± 7.55 | 0.06 |
| Large angles | 77.12 ± 17.4 | 88.14 ± 9.3 | < 0.05 |
Abbreviation: HRT, hand rotation task.
4.1.1. Response Time
By using the repeated measures analysis for group (CTL/SCZ) × rotation of stimulus angle (large/small), the main effect of stimulus orientation was significant (F (1, 34) = 10.50, P < 0.001), indicating that the subjects were longer in reaction time as stimulus was rotated further from the upright. In addition, the significant main effect of group was seen (F (1, 34) = 13.28, P = 0.001), indicating that patients responded significantly more slowly than the controls (Table 2). The interaction between group and stimulus orientation was not significant, suggesting a similar performance in the two groups across different orientations. Then, the two groups were compared for the mean scores of each angle of orientation, indicating significant differences in both large and small angles between the groups (P < 0.001).
4.1.2. Accuracy
Repeated measures analysis for response accuracy between the groups (CTL/SCZ) across the angles of the rotation of stimulus (large/small) showed the significant main effect of stimulus angle (F (1, 34) = 30.84, P < 0.001), indicating an increase in the error rate (less accuracy) with the response to more rotated stimuli in the two groups. The significant main effect of group (F (1, 34) = 5.01, P = 0.03) indicated that the performance of the patients was less accurate than the performance of the controls (Table 2). Then, the two groups were compared for the mean scores of each angle of orientation, and significant differences were found in the large (P < 0.001) but not in small angles.
The significant interaction between group and stimulus orientation was not seen, suggesting a similar performance in the two groups across different orientations.
4.2. Dot Test of Visuospatial Working Memory
The mean inaccuracy (cm) in the three delay conditions was calculated. A repeated measures ANOVA was conducted with group (SCZ/CTL) as the independent variable and mean inaccuracy in each condition (immediate, 10 seconds, 20 seconds, and 30 seconds) as the within-subject factor. The result showed the significant main effect of group (F (1, 34) = 9.58, P = 0.004), indicating the performance of patients was less accurate than that of controls. Post-hoc analyses showed the two groups were significantly different at 10 seconds, 20 seconds, and 30 seconds delay. Moreover, the main effect of the delay condition (F (1, 34) = 13.37, P = 0.001) was also significant, showing a decrease in accuracy with increasing duration of delay (Table 3). A significant interaction effect between delay and group was seen (F (1, 34) = 6.19, P = 0.01), suggesting that the two groups represented different changes in the preservation of information across the delay conditions (Table 3).
| SWM | Patients | Controls | P Value |
|---|---|---|---|
| Immediate, cm | 1.25 ± 1.24 | 0.69 ± 0.22 | 0.07 |
| 10 s delay, cm | 3.20 ± 2.95 | 1.07 ± 0.37 | 0.04 |
| 20 s delay, cm | 3.36 ± 3.19 | 1.09 ± 0.38 | 0.04 |
| 30 s delay, cm | 3.18 ± 2.89 | 1.04 ± 0.35 | 0.03 |
| Working memory deficit | 1.99 ± 2.7 | 0.38 ± 0.37 | 0.06 |
Abbreviation: SWM, Visio-spatial working memory.
aWorking memory deficit = (average of delayed recall) - (immediate recall).
4.3. Correlation
To examine the effects of the maintenance of spatial information on performance on HRT in schizophrenic patients, we calculated the correlation coefficients (Pearson’s r) between distance errors of SWM and behavioral measures of HRT (accuracy rate, response time) (Table 4). The result showed a significant correlation between distance errors in all delay recall of SWM and HRA (Table 4).
4.4. Effects of Psychotropic Drugs
To study the effects of patient clinical characteristics on their responses to HRT and Dot test of SWM, Pearson’s correlation coefficients were calculated between the measures of HRT and distance error and the duration of disease, chlorpromazine equivalent dose, SAPS, and SANS scores. The results demonstrated that there was no correlation between patients’ performance and patients clinical characteristics and the chlorpromazine equivalent dose (all P > 0.6).
5. Discussion
It seems that spatial working memory plays an important role in performing a mental rotation task. While evidence has shown that schizophrenic patients demonstrate dysfunction on both tasks of HRT and SWM, the question about the underlying role of SWM deficits has remained. The goal of the present study was to investigate the correlation between motor imagery ability and SWM function in patients with schizophrenia, assessed by the HRT and the Dot test of SWM, respectively. Two parts in the main results of the study can be seen. On the hand rotation task, while schizophrenic patients performed significantly slower and less accurate compared to the control group, they represented a similar pattern of performance as the normal subjects. On the test of SWM, both patients and controls had progressive deficits with increasing delay periods as measured by the mean errors in distance, but the patients made significantly more distance errors at all the three delays. Finally, impaired accuracy in the mental rotation was associated with a distance error in the SWM task in the patient group.
On HRT, consistent with previous studies, our results showed a similar performance for patients and the control subjects (similar effects of orientation), and their ability to mental rotation of stimuli was maintained. However, the patients showed slower response time and higher error rates compared to controls, similar to the findings reported in the other studies (7-10). These results propose difficulties in the accurately manipulating mental representation of hands in patients with schizophrenia.
In agreement with prior studies, we found impairments in SWM or passive maintenance of the location of stimuli in patients with schizophrenia (5, 6, 14, 15). Moreover, the results showed that SWM deficits were moderately correlated with error rates of HRT in patients with schizophrenia. Our findings might indicate that the difference in accuracy between patients and controls is because of impaired SWM. Supportive evidence comes from a proposal suggesting that SWM is crucial in the storage and manipulation of mental images; thus, its dysfunction would lead to the impairment of behaviors guided by internal representations such as mental rotation (7).
It should be noted that we used the HRT, which requires both online maintenance and manipulation of spatial representations of hands. In addition, we applied the Dot test of SWM to examine the passive maintenance of spatial information in working memory. Therefore, our finding indicates that impaired passive maintenance and failure to allocate attention to the relevant features of the internal representation might be the underlying mechanism of mental rotation dysfunction in the patients. However, since our SWM task did not examine the manipulation of information, future studies are required to use the SWM task in which subjects are required to manipulate stimuli during a delay period of working memory (16, 17).
The current findings should be considered in light of the study limitations. The participants’ number (patient and normal groups) was relatively small. A possible confounding factor was the effect of antipsychotic drugs. However, we did not found any correlation between chlorpromazine equivalent dose and either HRT performance or the Dot test of SWM. In addition, the adverse effects of anticholinergic drugs were seen on memory; thus, more investigations are needed to study the relationship between the dysfunction of mental rotation and cognitive ability in a group of drug-naive schizophrenic patients, as well as in different types of disease.
In summary, the results of this study replicated previous studies of dysfunction in motor imagery and visuospatial working memory in patients with schizophrenia. Moreover, the present study provides evidence that impairment in mental imagery is related to the impairment of SWM in patients with schizophrenia.