1. Context
Schizophrenia is a ubiquitous disease that concerns 1% of the world’s population (1, 2). It is a severe mental disorder characterized by a set of different symptoms varying in intensity: the most dramatic of which are delusions, hallucinations, mental dissociation, and, for the most handicapping, social withdrawal, denial of the body, and cognitive difficulties (3). In essence, schizophrenia means patients are not always able to perceive their health problems and this causes them to make the wrong decisions when it comes to resolving them on their own (4). This involves the coexistence of mental disorders with numerous organic co-morbidities, including the classic metabolic syndrome (diabetes, hypertension, dyslipidemia, and abdominal obesity) (5-7). Not to mention malnutrition, poor health practices, and antipsychotic treatments, which are the side effects that combine with all the co-morbidities to which they are exposed (8). Stigmatization and discrimination also play a role.
The poor oral health of patients with schizophrenia (PWS) and the resulting pathologies are more rarely mentioned (9, 10). Yet the data on the subject shows that the oral health of these patients is poor and the consequences are major: they impact their physical health, in particular by exposing them to the risk of cardiovascular disease, and alter their quality of life, their well-being, and their social functioning. In a recent review, Kiseley et al. found an important deteriorated state dental patient with severe mental disorders such as bipolar disorder, dementia, and schizophrenia (9). However, in their study, the authors didn’t individualize PWS from the others while they present distinctive clinical features and socio-professional differences. Moreover, most of the selected studies were conducted among hospitalized patients. It is therefore difficult to have a clear idea of or outpatient oral health monitoring of patients to date, especially since the last review specifically targeting the population of PWS, for Yaltirik et al. is more than 10 years (11). In this article, clinical features of schizophrenia have been reviewed and the important consequences from the perspective of dental practitioners have been highlighted; however, they didn’t describe the dental or periodontal index compared to the general population. Therefore, a recent review is needed to assess the magnitude of level of oral health among PWS, to have a better overview of the difficulties that these patients may encounter in the managing of their oral health, and to provide suggestions to improve it.
Our review of the literature aims to:
1) Estimate the frequency of teeth disorders in PWS.
2) Assess the level of treatment for teeth disorders.
3) Compare the number of clinical studies published for this population in relation to that of other chronic pathologies.
4) Determine the factors that influence the dental treatment of PWS
5) Suggest possibilities for improvement.
2. Evidence Acquisition
Our systematic review was conducted according to the recommendations of the Cochrane handbook for systematic reviews of interventions (12). The present report follows PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines (13).
2.1. Search Strategy
MEDLINE is the largest and most widely used biomedical bibliographic database in the world (14); therefore, we conducted a comprehensive literature search on MEDLINE via the PubMed database up to the 30th of June. In this search, we looked for all the papers on oral health and schizophrenia. For our search strategy, we used free-text searching of all relevant fields to retrieve the studies of interest. The text-word terms were selected based on the names mostly encountered in oral health and schizophrenia. We excluded editorials and reviews in order to select only original studies on the subject and we applied a set of inclusion and exclusion criteria.
2.2. Systematic Review
We applied a broad search strategy, including the keywords in the following formula: “Schizophrenia” AND (“dental care” OR “DMF Index” OR “Dental Care for Chronically Ill” OR “Oral Health” OR “Dental Health Surveys” OR “Periodontal diseases”).
Studies identified through database searches were first screened on the basis of their title and abstract. Studies were excluded if it was clear from the article title or abstract that the trial was not relevant or did not meet the inclusion criteria. Two independent reviewers (F.D. and B.T.) read the abstract and included studies according to their content. In case of disagreement, a 3rd reviewer made the decision after reading the title and abstract (J.C.C.G). Thus, full manuscripts were read to check the inclusion and exclusion criteria. We used a standardized form, according to the Cochrane collaboration (12).
In the present work, we aimed to review all studies that dealt with the oral health of PWS as well as studies that dealt with the promotion of oral health in this population. Studies had to fulfill the following criteria to be included:
- Clinical studies in the areas of oral healthcare, therapeutics, and epidemiology.
- In patients diagnosed with schizophrenia.
- From a homogeneous group of patients, including homogeneous subgroups of PWS if several pathologies are the subject of study.
- Be published in a peer-reviewed journal.
- Be in English.
Exclusion criteria included: reviews, editorials, case reports, comments, and preclinical studies.
2.3. Status of the Research
To evaluate the level of the research on oral health in PWS, we compared the number of articles published on the subject for this pathology with other chronic somatic pathologies. To analyze this parameter, we used the same keywords as in the formula above with, instead of “schizophrenia”, the following diagnostics: “rheumatoid arthritis”, “Parkinson’s disease”, and “systemic lupus erythematosus”.
3. Results
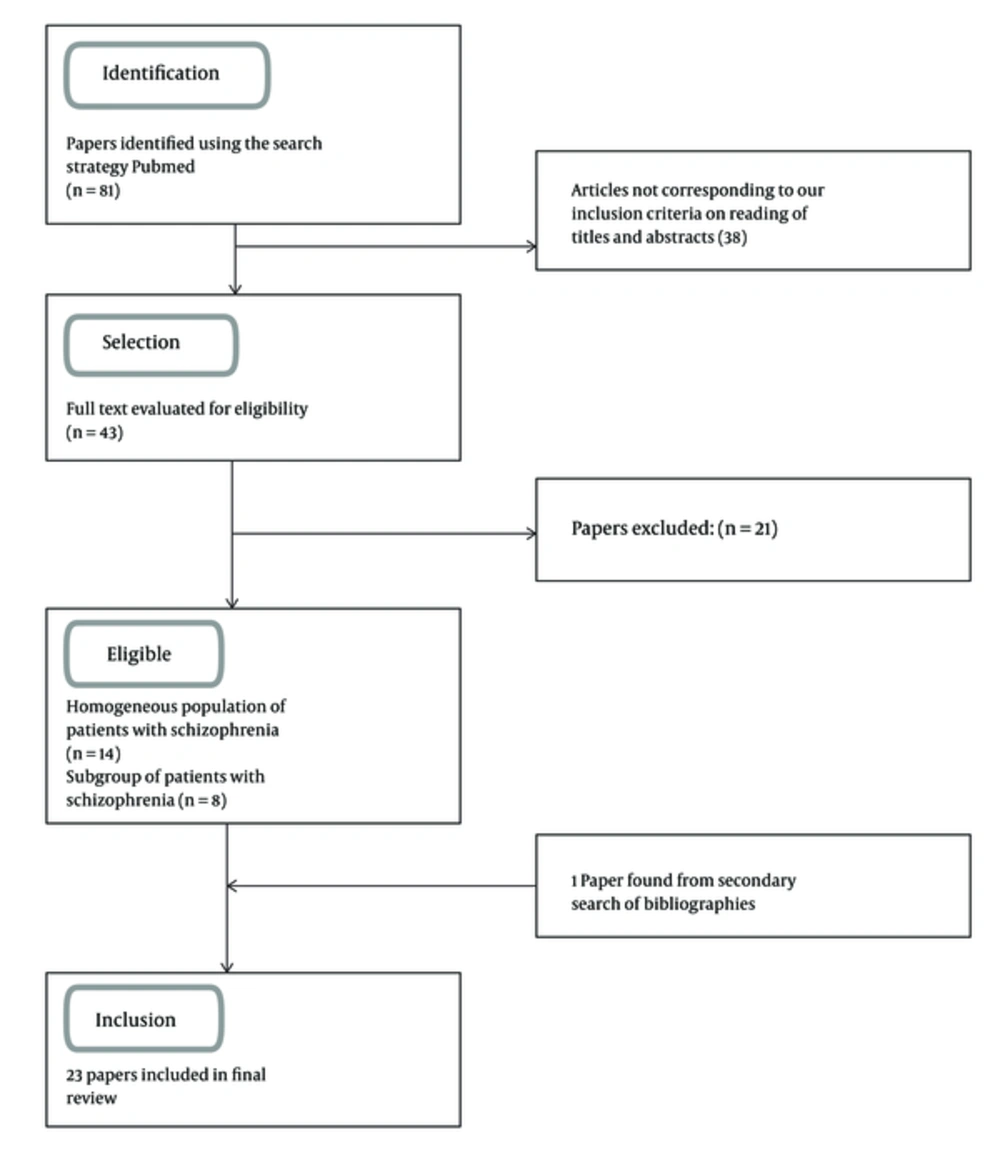
We found over 81 citations of interest in the initial electronic searches, of which 43 were potentially relevant and assessed for eligibility. Of the remainder, we excluded 1 that did not meet our inclusion criteria, however, we included 1 paper found from a secondary search of bibliographies, leaving 23 papers for formal review (15-25) (26-37) (Figure 1).
We identified 14 articles that dealt with dental status (16, 18, 20-22, 24, 26, 27, 30-33, 36, 37) 6 with periodontal status (15, 19, 22, 27, 31, 34), 7 with associated variables studies with oral health (18, 20, 26, 29, 30, 34, 35), 3 on the use of dental and somatic care (17, 28, 29), and 2 about oral health and quality of life (25, 35). Most of the studies we selected are from Europe and Asia (Table 1).
| Study | Year | Country | Outpatients or Hospitalized Patients | N | Average Age | DMFT | Periodontal Indexes | Other |
|---|---|---|---|---|---|---|---|---|
| Thomas et al. (15) | 1996 | Greece | Hospitalized | 249 | 50.3 ± 13.7 | 23.3 ± 8.4 | OHI-S3.8 ± 1.9 | DMFT and OHI-S correlated with BPRS |
| Velasco et al. (16) | 1997 | Spain | Hospitalized | 352 | - | 25.3 ± 7.4 | - | - |
| Dickerson et al. (17) | 2003 | USA | Outpatient | 100 | 42 ± 9.2 | - | - | Somatic Healthcare Utilization |
| McCreadie et al. (18) | 2004 | UK | Outpatient | 428 | 43 ± 13 | Number of teeth by age | - | Associated variables studies |
| Persson et al. (19) | 2009 | Sweden | Outpatient | 33 | Between 20 and 65 years old | 10.4 ± 6.2 | OHS = 3 | - |
| Chu et al. (20) | 2010 | Taiwan | Hospitalized | 1103 | 50.8 ± 10.8 | 13.9 ± 8.5 | - | Associated variables studies |
| Zusman et al. (24) | 2010 | Israel | Hospitalized | 209 | - | 24.3 ± 8.6 | - | - |
| Persson et al. (25) | 2010 | Sweden | Outpatient | 37 | Between 20 and 65 years old | - | - | Oral health quality of life (OHIP-14) |
| Gurbuz et al. (26) | 2010 | Turkey | Hospitalized | 339 | - | 20.8 ± 7.3 | - | Associated variables studies |
| Arnaiz et al. (27) | 2011 | Spain | Outpatient | 66 patients with schizophrenia 66 control patients | 40 ± 11.2 | 13.5 ± 7.3 7.8 ± 4.4 | CPI=2.3 ± 0.8 CPI = 1.0 ± 0.9 | PANSS-P correlated with lower DMFT (1) |
| Chu et al. (21) | 2011 | Taiwan | Hospitalized | 878 | 50.6 ± 10.9 | 13.9 ± 8.4 | - | BMI: 24.7 ± 4.2 |
| Janardhanan et al. (28) | 2011 | USA | Outpatient | 198 patients with schizophrenia and 113 patients for comparison group | Older (55 years old) patients with schizophrenia | - | - | Use of dental care |
| Nielsen et al. (29) | 2011 | Denmark | Outpatient | 21 417 for 2006, 18 892 between 2004 and 2006 | - | - | - | Use of dental care and Associated variables studies |
| Chu et al. (22) | 2012 | Taiwan | Hospitalized | 1103 | 50.8 ± 10.8 | 13.9 ± 8.5 | 35.9% CPI ≥ 3 vs. 5% control | - |
| Tani et al. (30) | 2012 | Japan | Hospitalized | 523 | 55.6 ± 13.4 | 18.8 ± 7 for 40 - 59 years | - | Associated variables studies |
| Eltas et al. (31) | 2013 | Turkey | Outpatient | 33 patients with hyposalivation-inducing medication | 31.8 ± 9.3 | 8.4 ± 5.6 | PI = 80 ± 39 | Salivary flow 0.23 mL/min |
| 20 patients with hypersalivation-inducing medication | 29.3 ± 9.7 | 8.2 ± 6.3 | PI = 58 ± 41 | Salivary flow 1.12 mL/min | ||||
| Nikfarjam et al. (32) | 2013 | Iran | Hospitalized | 123 | 38.8 ± 10.4 | 19.4 ± 7.7 | - | PANSS-N (2) correlated with higher DMFT 37.5% needed fixed prosthesis and 63.5% |
| Chu et al. (23) | 2013 | Taiwan | Hospitalized | 1103 | 50.8 ± 10.8 | - | - | needed removable prosthesis |
| Bertaud-Gounot et al. (33) | 2013 | France | Hospitalized | 59 | - | 14.6 ± 7.4 | - | DMFT patients with schizophrenia vs General population |
| Shetty et al. (34) | 2014 | India | Hospitalized | 250 | Between 25 and 55 years old | - | PI , correlated with duration of disease | Associated variables studies |
| Tang et al. (35) | 2014 | China | Hospitalized90% | 158 | 37.4 ± 12.4 | - | - | Associated variables studies and Oral health quality of life |
| Grinshpoon et al. (36) | 2015 | Israel | Hospitalized | 348 | 51.4 ± 14.5 | 23.5 ± 9.9 Vs 19.0 ± 10.5 | - | Typicals Antipsychotic Vs Atypicals |
| Wey et al. (37) | 2015 | Malaysia | Hospitalized | 543 | 54.8 ± 16 | 20.5 ± 9.9 | Only 1% Have healthy gums | Measure tooth decay and gum disease |
aValues are expressed as mean ± SD.
3.1. Frequency of Teeth Disorders in Patients with Schizophrenia
Decayed (D), missing (M), and filled (F) teeth (DMFT) index:
The DMFT index reflects a person’s lifetime experience of dental caries. Used by the world health organization, it is a dental index designed to both quantitatively and qualitatively measure the dental health of an individual. It corresponds to the sum of decayed (D), missing (M), and filled (F) teeth. The index is graduated from 0 (no decayed, missing, or filled teeth) to a maximum of 32, not counting wisdom teeth (38).
Among the articles selected in our review, we found that the DMFT index for hospitalized PWS varies from 8.39 to 27.17 according to age group. By comparison, the DMFT index for the general population, according to age group, is sometimes half that (16, 18, 20-22, 24, 26, 27, 30-32, 36, 37). However, a study conducted in France by Bertaud-Gounot et al. (33) did not find a difference between the DMFT index of PWS and that of the general population. When we look at the DMFT index values of the different studies selected, we notice they vary from one country to the other and that it is difficult to make statistical comparisons.
3.2. Periodontal Disease
Periodontal health is assessed using different indexes. The periodontal index, called the CPI (community periodontal index), is the world health organization’s (WHO) reference index (39). The CPI is used to measure the state of oral hygiene and periodontal disease by assessing 3 parameters (bleeding, subgingival calculus, and periodontal pocket depth) according to 4 levels of severity: 0 (absence of the disease) to 4 (most severe stage). In their study, Arnaiz et al. (27) compared the CPI index of patients in outpatient care with a control group. The authors found that the CPI was twice that of the PWS. Periodontal disease in PWS (CPI ≥ 2) is furthermore more severe than in the general population (CPI ≥ 1) (22, 27).
Others were used to assess the periodontal health of PWS:
The simplified oral hygiene index (OHI-S) corresponds to the sum of scores for plaque (from 0 to 3) and for calculus (from 0 to 3) assessed on the vestibular and lingual surfaces of 6 preselected teeth. OHI-S scores range from 0 to 6. For PWS, the OHI-S is assessed between 2.22 and 4.46 according to the age of these patients and the method of care given to them (15). In this study, there was no element of comparison available in the general population.
The plaque index (PI) scores 0, 1, 2, 6, and 8, which are the periodontal state of each tooth. For Eltas et al. (31), the PI is higher when antipsychotic treatments are antisialic rather than hypersalivation-inducing. A high PI score will be combined with the amount of time the mental illness has been present (34).
The oral hygiene status (OHS) corresponds to the plaque score (from 0 to 3) of each tooth proportional to the number of teeth present. By using the OHS, Person et al. (19) concluded that periodontal treatment needs are more significant in PWS than in those suffering from other mental disorders.
It is not easy comparing all the results of these studies since the indexes are different and don’t measure the same periodontal health parameters.
3.3. The Level of Treatment for Teeth Disorders in These Patients
We find that dental visits for patients in outpatient care are widely underutilized by PWS in relation to those of other medical specializations in the United States (17). The frequency of visits for PWS is lower than patients in the general population (15, 18, 28, 29, 35).
Dental care and prosthetic rehabilitation needs (removable or fixed prostheses) are substantial in relation to the general population. They were assessed with 1,103 patients hospitalized in Taiwan at 63.5% and 37.5%, respectively (23). In the United Kingdom, McCreadie et al. (18) found that 3% of PWS, between the ages of 16 and 44, are completely without teeth, while the same is true of less than 1% of the general population.
3.4. Status of the Research
Schizophrenia is a chronic disease that concerns 70 million people worldwide and only 23 conclusive studies on oral health were identified on PubMed for this disorder (Table 1). By comparison, the oral health of patients suffering from a chronic disease with a lower prevalence, such as rheumatoid arthritis, Parkinson’s disease, and lupus erythematosus, have been the subject of many more publications (Table 2). This number of publications adjusted to the level of the population of PWS shows a scientific production rate on the subject of oral health that is 3.73 times higher for rheumatoid arthritis, 5.65 times for Parkinson’s disease, and 6.52 times for lupus erythematosus. Apparently, the status of research on the oral health of PWS is insufficiently considered compared with other chronic pathologies.
| “Schizophrenia” | “Rheumatoid Arthritis” | “Parkinson’s Disease” | “Systemic Lupus Erythematosus” | |
|---|---|---|---|---|
| Prevalence of the disease in G pop, % | 1 | 0.3 | 0.1 | 0.04 |
| Number of clinical studies on oral health on PubMed | 23 | 26 | 13 | 6 |
3.5. The Factors that Influence the Dental Treatment of Patients with Schizophrenia
Generally, age, duration of mental illness, tobacco, alcohol, psychoactive substances, poor eating habits (diet high in sugar and low in fiber), poor dental hygiene (frequency and effectiveness of brushing, use of fluorides), low socio-economic, and cultural determinants are risk factors common to tooth decay and periodontal disease (15, 18, 20, 27, 30, 32). These factors are aggravated by stigmatization and discrimination, which is why PWS are not subject to the same amount of attention, in terms of their physical health, as others (40).
Dental hygiene, among patients suffering from severe mental disorders, seems to be worse in PWS (19). The negative symptoms of schizophrenia are associated with a DMFT and a high CPI and are linked to less frequent dental visits (15, 18, 27, 28, 35).
For a long time, PWS were thought to be insensitive to pain. Recent studies show this to be false (41). Delays in diagnoses are sometimes observed in relation to, on one hand, the confusion in reading pain signals when they are lost in the symptoms of the schizophrenia and on the other hand, the absence of patient verbalization of pain. This absence of reaction could be caused by indifference to pain rather than insensitivity to it (42). In this case, it very often generates patient indifference to "somatic disorders" and to treatment delays detrimental to them (43).
Being underweight could be associated with an increased risk of tooth decay in the population of hospitalized PWS (21).
Antipsychotic and anticholinergic treatments (hyposalivation) increase the risk of tooth decay (20, 29) and the prevalence of periodontal diseases (31). Some treatments can cause gingival pain (ziprazidone) (44) and problems with the temporomand ibular joint (trismus) (45). Generally, schizophrenic patients with atypical antipsychotics have better dental health than patients treated with typical antipsychotics or a combination of both (37).
The interest in assessing the quality of oral health is recent in PWS. Good oral health could be associated with the number of teeth present. Men would consider dental care isolated from their health issues while women integrate it in their general health. The interest PWS could have in their oral health could depend on the symptoms of the disease and their intensity (25). Tang et al. (35) didn’t notice any major difference between the scores of the oral health quality scale oral health impact profile with 14 items (OHIP-14) between patients suffering from schizophrenia and other severe mental disorders (Bipolar and major depressive disorders).
4. Discussion
The aim of this study was to access the level of oral health and the level of research about PWS. We found that the dental and parodontol index in PWS are double of the general population. However, this result should be interpreted with caution. Indeed, it’s difficult to make a comparison between the 2 populations using the selected studies in the present review because the ages of the population are often different and the periodontal health is valued with different index. Moreover, beyond the assessment, the challenge to improve the oral health status in PWS is to determine appropriate programme of measures for this population.
4.1. Proposals for Improving the Treatment of Patients with Schizophrenia
4.1.1. Need for Early Screening
The symptoms of schizophrenia reduce the capacity to plan and execute such routine personal hygiene activities as those of dental hygiene. These disorders are caused by a disruption of the executive functions. It should also be noted that the lack of context analysis and deficiencies of reasoning, linked to cognitive disorders, means the patient does not perceive the significance of their health problems and does not make the appropriate decisions to resolve them (46). In this context, 60% of people suffering from schizophrenia do not perceive their disorders and are therefore not motivated but are even opposed to taking care of them (47). Generally speaking, disturbances in thoughts linked to mental illness mean that the patient has no perception of these needs and is therefore late going out and getting treatment (4). In this case, it’s important to anticipate by screening early for health problems since the intensity of cognitive disorders is associated with a lack of initiative (48). The most severely ill patients will therefore not spontaneously go and get treatment and will not be very motivated to change their behavior to develop good personal and dental hygiene (49). Nielsen et al. (29) reported that 43% of the PWS, polled out of 21427, declared they visited their dental surgeon during the previous year versus 68% in the general population.
4.1.2. Promote Access to Care
PWS have less access to healthcare systems (17, 18, 28, 29). In Denmark, Nielsen et al. (29) showed that, for hospitalized patients with schizophrenia, approximately 30 days were needed to get an appointment with a dental surgeon (29). Other than time issues, we also observe a poor use of the healthcare system by these patients, which explains the delay in visits and the lack of visits and follow-up. This insufficiency can be explained by the cost of dental care, which is given as one of the reasons for limiting access of vulnerable populations to healthcare (49, 50). Scarce financial resources also widely contribute to a lack of access to the healthcare system (49). This limit is even more far-reaching as oral care needs are significant in these populations. Moreover, these patients prefer hospital emergency services, which are more accessible than the private healthcare system (51).
Therefore, generally speaking, healthcare systems have a poor capacity to meet their specific needs (10, 52).
4.1.3. The Partner Patient Concept
In response to the increase in chronic diseases, the very high rate of treatment non-observance and the need for more patient autonomy, the partner patient concept is gradually emerging as a healthcare approach (35-54). The experiential knowledge of patients in psychiatry was used to establish the charter of citizenship, which opens up prospects in terms of healthcare system reorganization (55, 56). The international program for participatory-action research (IPPAR), of which one of the missions is to promote the experiential knowledge of users, could develop in the form of a therapy education program that encourages restoring civic participation in mental health (57). This renewed patient capacity would, in this case, be used to improve the physical health problems of patients suffering from such severe mental disorders such as schizophrenia. The expertise of partner patients would be used to build family physician and dentist awareness of the specific health issues encountered by PWS so that they too can access the general healthcare systems.
4.1.4. The Need for Multidisciplinary Treatment
Smoking, side effects of antipsychotic treatments (hyposalivation), and socio-economic factors (low financial resources, insecurity) are major risk factors (10, 20, 31, 35). Poor oral health can also affect the quality of life through the social and psychological impact of deterioration in smile aesthetics (25, 27, 31, 35). There are also the neurological effects of first-generation antipsychotics (FGAs) (dystonia, dyskinesia), which produce shaking and prevent effective brushing, alter chewing and swallowing, and can be associated with aspiration problems (58). FGAs have metabolic effects, which are associated with poor oral health (8). The existence of a relationship between metabolic disorders and oral deficiencies has gradually taken hold over the last 10 years with, on one hand, the development of knowledge on the pathophysiology of the syndrome and its consequences on cells and tissues and, on the other hand, the observation that certain infectious diseases in the oral sphere, such as periodontal disease, had already been associated with each of the components of the metabolic syndrome. This means that diabetes is a risk factor likely to contribute to the development of periodontal disease. Control of the periodontal infection would furthermore contribute to control the diabetes (59, 60). Periodontal disease would also be a risk factor for cardiovascular diseases (ischemic heart disease) (61) and be associated with the pathogenesis of schizophrenia (34).
All the co-morbid disorders suffered by these patients, that is, dysmetabolic disorders, dietary deficiencies by non-observance of hygiene and dietary rules, the side effects of certain treatments (Phenothiazine), such as neutropenia by agranulocytosis (62, 63), addictive behavior (alcohol, drugs, etc.), smoking, risky behavior, overexposure to HIV, and HCV, insecurity, mean that these patients are predisposed to a more fragile immune system and higher risk of infection(64, 65). This contributes to the development of tooth decay and periodontal disease. That said, the attachment of germs of the oral origin to different organs, especially when the immune system is deficient, can have serious consequences in case of a brain abscess or infective endocarditis, which is probably underestimated in these populations (66, 67).
4.1.5. The Importance of Therapeutic Education
It is however possible to act successfully, by looking for patient collaboration in healthcare programs through motivational approaches (68). They deal with ways to help patients with their smoking, their sedentary lifestyle, their unbalanced diet, and their dental health status. This requires training people with cause to interact frequently with them, especially the family circle, social and medical social services personnel, private nurses, and general practitioners (18, 69, 70). Trials are underway, in Great Britain especially, and they are aimed at improving the oral health of PWS (primary evaluation criterion: number of visits to the dentist in the 12 months of the program, evaluation of the plaque score, frequency of daily brushing) (71), but also in France where a clinical trial with patients suffering from schizophrenia aims to build a program that promotes oral health and evaluates it (evaluation criterion: development of the CPI index: ClinicalTrials.gov Identifier:NCT02512367). Almomani et al. (72) showed that patients suffering from severe mental disorders could improve their oral hygiene.
4.1.6. Caregiver Training
An aversion of healthcare professionals vis-a-vis these patients (fear of the mental illness, lack of training) that leads to numerous biases and generates an insufficiently helpful attitude, but also the lack of initial training in this field contributes to caregiver misunderstanding and a stigmatization of patients suffering from schizophrenia (73). It is therefore important, in addition to building awareness of oral health problems in PWS, to train these caregivers on psychiatric pathologies. This training must foster a better understanding of patient symptoms in order to avoid misunderstandings regarding their behavior. It’s true, PWS sometimes present apathy, a lack of initiative, and repeated forgetfulness linked to their psychiatric pathology (74). Problems with executive functions can also explain why these patients have a difficult time getting to a given place at a given time. Other symptoms, such as anxiety, the fear of public transportation, or even scarce financial resources are factors that contribute to the lack of access to the healthcare system (49).
Recommendations regarding best practices exist and caregivers must be familiar with them. These include those of “the British Society for Disability and Oral Health” meant to optimize the treatment of patients suffering from severe mental disorders (75). They are based on easy to access dental practices, facilitated financial support for healthcare, multidisciplinary treatment of dental disorders, prospective patient support, and development of professional training. However, they are based on expert consensus not on evidence based on facts (76).
4.1.7. The Level of Research on Oral Health in PWS Must be Improved on Both a Qualitative and a Quantitative Level
Having detailed and up-to-date information available is essential in understanding the incidence of oral health in PWS as well as observing treatment results and the quality of care. Aimed at patients suffering from severe mental disorders, Kisely et al. (9) (evaluation criteria, edentulism, and the DMFT index) showed that a significant number of publications on the subject lacked specifics in the diagnostic criteria of psychiatric pathologies, comparison groups, or presented methodological inaccuracies. When we look at an homogeneous group of PWS, the studies are also of variable quality. Among the 21 studies chosen, 4 were conducted by Chu et al. (20-23) in Taiwan on the same population. In all the studies, patient inclusion is done prospectively and not randomly, by random drawing, with occasionally a modest workforce, which limits the sample size of results (27, 31). We can therefore assume that the published data only partially reflects the oral health status of these populations. It’s true, patients who do not agree to participate in these studies are likely to be unwilling to get a dental work up because of their poor oral health (19). Moreover, most of the studies are conducted with hospitalized patients while most PWS are in outpatient care (77): they only report on oral health for a portion of the population of PWS. The qualitative research focuses very little on the field of schizophrenia and oral health. The issue regarding the poor oral health of PWS was brought up by Persson et al. (25) and Tang et al. (35) by exploring the representations of this health problem. The oral health quality scale used in the protocol for these studies (OHIP-14) is an auto-questionnaire validated in the general population (78). We had some concerns regarding the psychometric validity of this scale in terms of PWS.
4.1.8. Oral Health Prevention and Promotion Programs
Oral health prevention and promotion programs, based on knowledge in the general population and transposed for patients with severe mental disorders, did not prove to be very effective (5, 76). The particular profile of patients suffering from schizophrenia must be taken into account. Khokhar et al. (76) specify there is no study with a high level of proof to support the direction of current practices in this field.
4.1.9. Evaluation of Quality of Life
Improvement in the quality of life during a somatic treatment must be assessed according to actual experience of mental disorders. The interest shown by PWS in the construction of the quality of life scale in the form of auto-questionnaires is a recent phenomenon. The difficulty involved in constructing these scales resides in the capacity of patients suffering from schizophrenia to document their health status due to the presence of cognitive disorders and psychotic characteristics (35, 79, 80). Lancon et al. however, showed that the auto-questionnaire Schizophrenia quality of life (S-QoL), constructed based on semi-structured interviews with patients suffering from schizophrenia (hospitalized, outpatient treatment, in acute phase and stabilization phase), made it possible to assess the subjective quality of these patients and to show a wide divergence between the points of view of the patients and those of outside assessors (81).
Other than the need to assess quality of life according to actual experience of mental disorders and their evolution, the assessment of somatic care needs is substantial in these populations. In the general population, oral health quality assessment tools were built by North American teams. The geriatric oral health assessment index (GOHAI) (82) and the oral health impact profile with 14 items (OHIP- 14) (78) were designed for adults and were validated in the general population and the oral impact on daily performance (OIDP) (83) was designed for children. As far as we know, there are no oral health quality scales validated for PWS.
5. Conclusions
Since the last study of Yaltirik et al. in 2004, the oral health of PWS has not evolved. We always note the significant differences between oral health indices of PWS and those of the general population. Research in the field of oral health in PWS doesn’t seem to mobilize researchers. However, 70 million people are concerned by the deterioration of their oral health in connection with their schizophrenic disorders. This issue is a major public health issue. Better taken into account, it must contribute to the overall improvement of quality of life and the general health of these subjects. Simple measures are possible, such as information on the side effects of treatments and regular somatic monitoring. For this, specific awareness-for building tools for patients and healthcare professionals must be created. Efforts need to be made to reconcile knowledge on the psyche, somatics, dentistry, and medicine, all within the scope of a global approach. When organized and supported to this end, partner patients can actively and effectively contribute to these efforts.