1. Background
The postpartum period is the time with the greatest risk of mood disorders including depression and psychosis. Postpartum depression is a clinical syndrome that is more severe than postpartum blues and affects the families more strongly. This syndrome is a combination of physical and emotional sickness and behavioral changes that some women experience after the birth of their children (1). Approximately, 40% of the four million women who give birth each year experience various forms of postpartum mood disorders (2). Annually, about 44 million pounds are spent on the treatment and care of patients with mood disorders and postpartum depression in Britain (3). The prevalence rate of postpartum depression has been reported as 10% - 15% worldwide (4-8). In the studies conducted in Iran, postpartum depression has shown a high prevalence. For instance, in research by Sadat et al. (9) and Habibzadeh et al. (10), the prevalence of this disease was reported as 39% and 27%, respectively. Postpartum depression can be accompanied by a decrease in mothers’ activities and disturbance in the relationship between mother, child, and family. Persistence of this disorder and lack of its timely diagnosis and treatment can lead to an inefficient adjustment of mothers to their children, spouses, or families, resulting in the inability to perform the role of mother and wife. In severe cases, it can lead to the mother’s suicide or infanticide (11). In about 25% of cases, this disorder turns to permanent depression or may move toward psychosis, if left uncured (12). The low diagnosis rate (about 50%) limits the appropriate treatment of women with this problem (13). In contrast, if mothers who are at risk of depression can be diagnosed, their depression can be prevented (14). Appropriate screening in this regard requires the identification of risk factors. A wide range of causes has been suggested for the etiology of postpartum depression (1).
There is evidence that oxidative stress plays a role in more than 100 diseases including cancer, neurodegenerative diseases, and most psychiatric problems (15). Antioxidants are elements that protect the body from oxidative stress. The antioxidant defense system includes a series of enzymatic (like catalase) and non-enzymatic (like albumin, uric acid) components. The plasma of human blood contains a large number of antioxidants with synergistic effects; thus, measuring each of these elements alone cannot express the status of antioxidants. Therefore, the measurement of total antioxidant capacity can give some information on the antioxidant status of a person.
Oxidative stress can lead to lipid peroxidation. Malondialdehyde (MDA) is a final product of lipid peroxidation and one of the most common factors for checking the status of oxidative stress (16). Few studies have been conducted on oxidative stress in depressive disorder, with contradictory results (17). The literature examining the possible relationship between oxidative stress during pregnancy and postpartum depression remains limited. To the best of our knowledge, research only has focused on depression among non-pregnant women and associations between postnatal dietary patterns and postpartum mental health status. However, the data from clinical studies are not so unequivocal.
2. Objectives
The goal of this study was to determine the relationship between oxidative stress during pregnancy and postpartum depression in women referring to the health centers of Shahid Beheshti University of Medical Sciences in 2017.
3. Methods
This study was conducted with a prospective analytical design from January to May 2017. The study protocol was approved by the Ethics Committee of the School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences (IR.SBMU.ries.Rec.1390.98). The sample was selected among patients referring to the health centers affiliated to Shahid Beheshti University of Medical Sciences during the third trimester of pregnancy and the same women were selected two to six weeks after delivery. It was a multistage sampling; at the beginning, the sample was proportional to the size of each class from each of the health centers of Shahid Beheshti University of Medical Sciences (east and north); then, a number of centers were randomly selected and each center was considered a cluster. Finally, from among the selected centers (Salavati, 12th Bahman, and Taleghani), purposeful sampling was conducted to obtain the required sample size. In this study, the number of samples was estimated as 61 women considering previous studies (1) that stated the prevalence of postpartum depression as 10% - 15% with 95% confidence interval and 5% alpha error. With a 10% possibility of participants’ loss in the follow-up after delivery, 80 people were considered suitable for this study.
To collect the data, we used a demographic and midwifery questionnaire, the Beck Depression Inventory, Edinburgh Postpartum Depression Test, and Stein’s Postpartum Blues Questionnaire. Content validity was determined to establish the validity of the demographic and midwifery questionnaire.
The Edinburgh Postpartum Depression Test was formulated by Cox et al. Its validity for screening postpartum depression was investigated in several studies. Considering a score of 10 or more for evaluating postpartum depression in the Edinburgh Postpartum Depression Test, various studies have determined the sensitivity of 84% - 100% and specificity of 82% - 84% (18). Its validity has also been confirmed by Mazhari in Iran (19).
The Beck Depression Inventory includes 21 multiple-choice questions with scores of 0 - 3. The scores ≤ 9 represent non-depressive people, 10 - 12 show the people at risk, 12 - 14 include those who should refer to consulting centers, and above 14 show depressed people. This test was validated using the construct validity in Iran by Mazhari with a correlation coefficient of 0.87. This test had a sensitivity of 0.87 and a positive predictive value of 73% (19).
The test-retest was used to determine the reliability of the demographic and midwifery questionnaire, with the r value of more than 0.80 as acceptable. In this study, the r value was more than 0.9.
The internal consistency method or Cronbach’s alpha was used to determine the reliability of the Beck Depression Inventory, Stein’s Postpartum Blues Questionnaire, and Edinburgh Postpartum Depression Test, which showed the R values of 0.89, 92.91, and 0.0, respectively. In the study conducted by Sadat et al., Spearman correlation coefficient for measuring Edinburg reliability in repeated measures was 0.92.
A test-retest method was used to determine the reliability of Stein Questionnaire; 10 women who were between 3 and 10 days after delivery were studied in two phases with a 2-h interval and the correlation between the results was determined (r = 0.9).
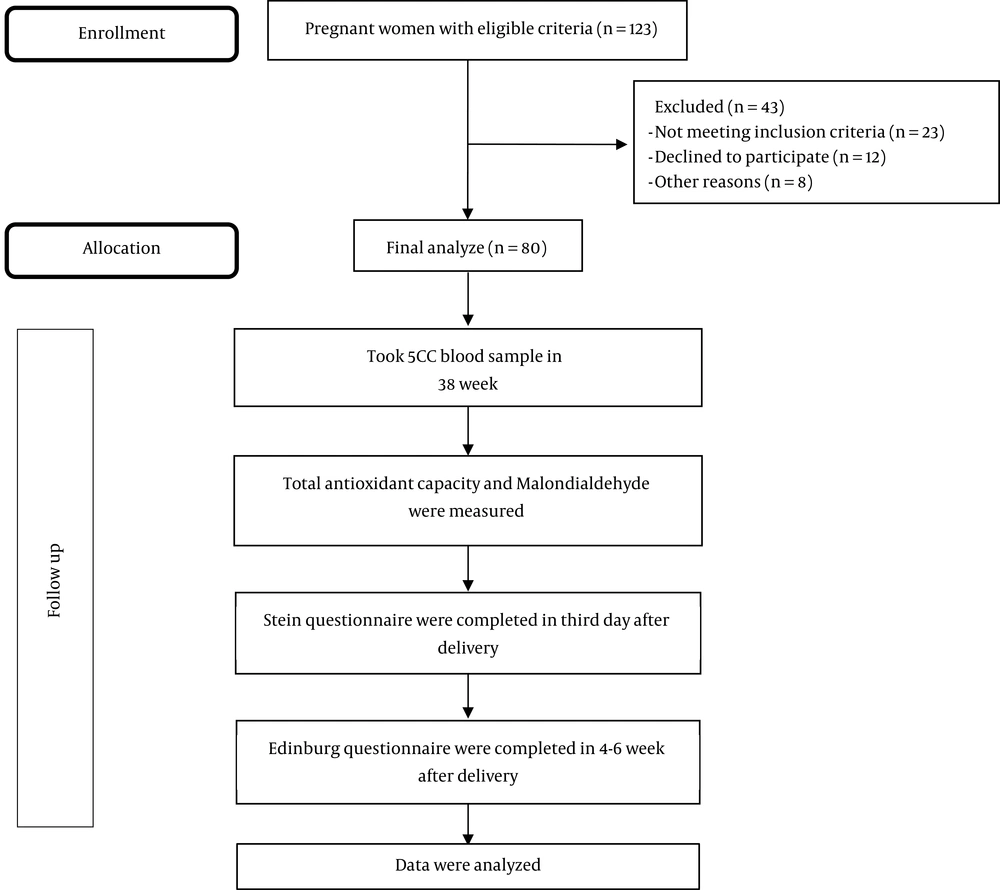
All the women participating in this study had the following criteria: they were Iranian, literate, and singleton pregnant, with the gestational age of 38 - 40 weeks, maternal age of 18 - 35 years, vaginal delivery or uncomplicated cesarean section. The exclusion criteria included a history of depression during a previous pregnancy or during the life, history of infertility, suffering from chronic or acute diseases before or after delivery, vegetarian diets, and recent nervous tension such as the death of loved ones. A total of 123 pregnant women were recruited. Among them, 23 pregnant women for not meeting the inclusion criteria, 12 for declining to participate, and eight participants for other reasons were excluded from this trial. Thus, 80 pregnant women finally remained for analysis (Figure 1).
Before completing the questionnaires, the nature and objectives of the research and free participation in the study were explained and written consent was obtained from the participants. The demographic and midwifery questionnaire and the Beck Depression Inventory (to reject depression before and during pregnancy) were completed for all the research units at weeks 38 - 40 of pregnancy. The depressed women were excluded from the study. At the same time, 5 cc venous blood was taken and the total antioxidant capacity and MDA were measured by an atomic absorption device using the atomic absorption method in the Research Laboratory for Endocrinology and Metabolism, Shahid Beheshti University of Medical Sciences, and the results were reported as µmol/L. In this study, an atomic absorption apparatus (model CTA-2000 made by Kamtech Industries, Netherlands) was used. At the beginning of each work period, the device was calibrated using the zinc standard solution (100 µg/dL). Then, Stein’s Postpartum Blues Questionnaire was completed for all the subjects on the third day after delivery (when the women referred to perform the infant’s routine thyroid test). Around 4 - 6 weeks after the birth, when the participants referred for routine care, the Edinburg Depression Questionnaire was filled out and the score of above 10 indicated postpartum depression. The levels of postpartum blues and postpartum depression and their relationship with the level of serum oxidative stress were measured. It should be noted that all the research units received routine supplements during pregnancy and after delivery.
In this study, SPSS 18 software was used for data analysis. The chi-square test was used to compare qualitative variables and the t test and Mann-Whitney test were applied for comparing the ranked variables. Also, linear regression was used to examine the relationship between postpartum depression and oxidative stress.
4. Results
In this study, the relationship between oxidative stress and postpartum depression was studied among women referring to the health centers affiliated to Shahid Beheshti University of Medical Sciences. A total of 113 women were recruited in this study; however, 33 of them were excluded; 24 women had missing data (six for missing oxidative stress measurements and 18 for incomplete questionnaires) and nine women refused to participate. Finally, 80 participants were entered into the further analysis (Figure 1).
The mean age of the participants was 26.36 years with a standard deviation (SD) of 5.34 years. The mean age of the husbands was 31.45 with an SD of 5.70 years. Using the t test, we did not find any significant difference in the husbands’ age between the two groups (P > 0.05). The t test did not show any significant differences in terms of the duration of marriage between the two groups (P > 0.05). Moreover, 96.3% of women in the depressed group and 95% in the non-depressed group were in their first marriages. The t test showed no significant difference between the two groups (P > 0.05). In addition, 47.5% of women in the depressed group and 48% in the non-depressed group had one child and the t test showed no significant difference between the two groups (P > 0.05). It was also shown that 47.5% of women in the depressed group and 48% in the non-depressed group had two pregnancies and the t test showed no significant difference between the two groups in terms of the number of pregnancies (P > 0.05) (l). l). There was also no statistically significant difference in demographic characteristics between the two groups, as can be seen in Table 2, and both groups were similar (P > 0.05) (Table 2).
| Variable | Depression (N = 22) | No Depression (N = 58) | P Valuea |
|---|---|---|---|
| Age, y | 27.32 (5.9) | 26 (5.09) | > 0.05 |
| Husband age, y | 32.5 (5.6) | 31.05 (5.72) | > 0.05 |
| Marital age, y | 5.1 (5.8) | 4.5 (3.4) | > 0.05 |
| Child numbers | 0.8 (1.2) | 0.6 (0.7) | > 0.05 |
| Pregnancy numbers | 1.6 (0.7) | 1.7 (1.2) | > 0.05 |
| Marital numbers | 1 (0.2) | 1.1 (0.2) | > 0.05 |
Distribution of Women with and Without Depression: Obstetric Characteristics
| Variable | Depression (N = 22) | No Depression (N = 58) | P Valuea |
|---|---|---|---|
| Education (high school) | 11 (56.9%) | 27 (55%) | > 0.05 |
| Job (housewife) | 19 (80.4%) | 36 (62%) | > 0.05 |
| Husband education (high school) | 12 (62.5%) | 31 (59%) | > 0.05 |
| Husband job (employer) | 11 (56.9%) | 27 (55%) | > 0.05 |
| Monthly income (400,000 - 6,000,000 Rials) | 13 (59.1%) | 38 (65%) | > 0.05 |
| Housing situation (rent) | 11 (56.9%) | 26 (51.2%) | > 0.05 |
Distribution of Women with and Without Depression: Demographic Characteristics
The results indicated that 81.8% of pregnancies in the depressed group and 82% in the non-depressed group were wanted and the chi-square test showed no statistically significant differences between the two groups (P > 0.05). The chi-square test showed that the two groups had no significant differences in terms of the gender of their babies (P > 0.05). In the non-depressed and depressed groups, 95.5% and 98.3% of women were satisfied with the gender of their babies, respectively, and the chi-square test showed no statistically significant differences between the two groups (P > 0.05). In the depressed and non-depressed groups, there were 72.7% and 73% natural vaginal deliveries, respectively, and the chi-square test showed no statistically significant difference between the two groups (P > 0.05). In the depressed and non-depressed groups, there were 90.9% and 91.4% exclusive breastfeeding, respectively, and the chi-square test showed no statistically significant difference between the two groups (P > 0.05) (Table 3).
| Variable | Depression (N = 22) | No Depression (N = 58) | P Value |
|---|---|---|---|
| Wanted pregnancy | 18 (81.8%) | 46 (79.3%) | > 0.05 |
| Infant gender (boy) | 12 (43.3%) | 28 (48.3%) | > 0.05 |
| Favorable gender (mother) | 21 (95.5%) | 57 (98.3%) | > 0.05 |
| Favorable gender (father) | 19 (86.4%) | 52 (89.7%) | > 0.05 |
| Delivery mode | 16 (72.7%) | 42 (73%) | > 0.05 |
| Exclusive breastfeeding | 20 (90.9%) | 53 (91.4%) | > 0.05 |
Distribution of Women with and Without Depression: Obstetric Characteristics (Recent Pregnancy)
On the third to 10th day after delivery, 47.7% of women who referred to the health centers were suffering from postpartum blues. At four to six weeks after delivery, 29.2% were depressed. The incidence of depression was 78.6% in women suffering from postpartum blues and 9.8% in those free from postpartum blues and the chi-square test showed a significant relationship between depression and postpartum blues (P < 0.001) (Table 4).
| Postpartum Depression | Postpartum Blues | Test | |
|---|---|---|---|
| Patient | Non-Patient | ||
| Patient | 39 | 19 | P < 0.001 |
| Non-patient | 5 | 17 | RR = 2.9 |
| Total | 44 | 36 | CI = 1.34 - 6.52 |
Distribution of Women with/Without Depression and with/Without Postpartum Blues
The mean concentration of total antioxidant capacity was 0.98 µmol/L in depressed patients and 1.06 µmol/L in the non-depressed group. The t test showed no significant difference in terms of total antioxidant capacity between the groups (P < 0.01). The linear regression test showed that the risk of suffering from depression increased by one unit with each 0.13 unit reduction in the total antioxidant capacity. The mean MDA levels were 4.8 µmol/L and 4.2 µmol/L in the depressed and non-depressed groups, respectively. The t test showed no significant relationship between the two groups (P < 0.05) (Table 5).
| Variable | Depression (N = 22) | No Depression (N = 58) | P Valuea |
|---|---|---|---|
| Total antioxidant capacity | 0.98 (0.08) | 1.06 (0.12) | P = 0.01; CI = 0.01 - 0.14 |
| Malondialdehyde | 4.8 (1.17) | 4.28 (1.34) | P > 0.05 |
Distribution of Women with and Without Depression and Oxidative Stress Level
5. Discussion
The present findings support that high levels of oxidative stress can be associated with postpartum depression. The prevalence of postpartum depression in the fourth to sixth weeks was 27%. In a study conducted by Wojcik et al. (13), it was reported that the prevalence of depression was 29%, which was almost in line with the present work.
In this study, 78.6% of women with postpartum blues and 9.8% of women without postpartum blues suffered from depression. The incidence of depression was 2.9 times higher in the grieving group than in the non-grieving group (P < 0.001, CI = 1.34 - 6.52). Studies have demonstrated that the probability of depression and major depression increases by 85% in women suffering from postpartum blues (1).
The present study showed a significant relationship between suffering from postpartum blues and depression. The obtained results were in line with the study conducted by Rek et al. (20), which expressed that 81.8% of mothers with depression experienced postpartum blues. Also, in a study conducted in Tehran by Wojcik et al. (13), there was also a significant relationship between postpartum blues and depression so that the relative risk of depression was 7.3 times higher among afflicted women than among non-afflicted ones.
Depression also is associated with other issues such as prenatal depression, being a divorced woman, mother’s low age, medical problems in pregnancy, and a history of emotional and psychological disorders (13). In this study, the two afflicted and non-afflicted groups were similar in terms of these variables.
Another factor that may be effective in depression is the level of serum oxidative stress. Malondialdehyde, a final product of lipid peroxidation, is the most common oxidative stress that is being assessed in studies (16). In the present study, there were no significant differences in terms of MDA levels between the depressed and non-depressed groups. The result of this study was not in line with the results of Sarandol et al. (16), Baek et al. (21), Cumurcu et al. (22), and Vavakova et al. studies (23). They stated a significant difference in the MDA plasma level between the two depressed and non-depressed groups (P < 0.05). The reason for the differences can be attributed to blood sampling from non-pregnant people. In previous studies, blood samples were taken from non-pregnant individuals, but in this study, blood samples were taken during pregnancy. Lipid peroxidation decreases during pregnancy due to the increased demand for energy and increased fat metabolism and the body shifts from carbohydrates to fats (18). This leads to the decreased production of oxidative stress, which is the product of lipid peroxidation (16).
Antioxidants are substances that protect the body against toxic effects of oxidative stress. Oxidative stress plays a role in more than 100 diseases including cancer, neurodegenerative diseases, and psychiatric problems (24). The levels of antioxidants in the body can be assessed by the total capacity of serum antioxidants (16). The present study showed that total antioxidant capacity was lower in patients with postpartum depression than in the non-depressed group (1.06 versus 0.98 µmol/L). Linear regression showed that the score of postpartum depression increased by one unit with each 0.13 µmol/L decrease in total antioxidant capacity.
In a study by Cumurcu et al. in Turkey (22), there was a significant difference in total antioxidant capacity between patients with major depression and non-depressed participants (P = 0.0001, OR = 0.55). The results of that study were similar to the present study results. Vavakova et al. (23) and Barata et al. (25) also obtained similar results.
In a study conducted by Beak et al. (21), there was no significant difference in total antioxidant capacity between patients with major depression and non-depressed ones. The reason for this difference between the results can be probably that Beak et al. evaluated the total antioxidant capacity using a spectrofluorometric device and fluorescent filters. However, in this study, an atomic absorption device and atomic absorptiometry methods were used to evaluate TAC. The accuracy of the atomic absorption device for measuring and evaluating TAC is much more than that of fluorescent filters and spectrofluorometric device.
It should be noted that one of the strengths of this study was its cohort design. Additionally, confounding variables were controlled for or matched in both groups. Validity and reliability of the instruments were assessed and reliable and valid methods were used to control all the factors that might cause errors such as data collection, which was done by a midwife, or conducting the tests, which all were performed by laboratory experts. In the present study, total antioxidant capacity and MDA levels were measured in connection with postpartum depression only during pregnancy (weeks 38 to 40); this measurement can be done in different trimesters of pregnancy and in different periods after delivery.
In conclusion, our results showed that oxidative stress plays an important role in the pathogenesis of postpartum depression and suggests the importance of antioxidants use in diet among high-risk pregnant women. Nevertheless, further studies are necessary to confirm the physiological relevance of these results.