1. Background
Schizophrenia, affecting around 1% of the population, is a severe disorder involving chronic or recurrent psychosis and long-term deterioration in functional capacity (1). The characteristic onset of schizophrenia in early adulthood, its lifelong course, and debilitating symptoms, plus its high burden on caregivers and the society make it one of the most disabling and economically catastrophic disorders (1). Despite the improvement of antipsychotic medications, as the main-stay of treatment for patients with schizophrenia, the symptom response is not always optimal, side-effects are frequent, and treatment adherence is often poor (2-4). Therefore, still a large number of studies are ongoing for identifying new drugs of different classes that may be effective in the treatment of schizophrenia (2).
Zinc (Zn) is the second most abundant trace element in the body after iron (5). It is essential for over 300 processes such as enzymatic catalysis, gene transcription, gene expression, DNA replication, protein synthesis, tissue repair, and hormonal storage (6, 7). Zinc is also introduced as an antioxidant element (5, 8). It is required for development of the brain and also for its function; Zn deficiency can cause neuronal damage (9-11). While Zn deficiency is reported in patients with Alzheimer’s Disease (AD), cognitive improvement has also been demonstrated in these patients after supplementation with Zn in some preliminary studies (11). Some studies, however, few in number, have also shown the efficacy of Zn in improvement of Attention Deficit Hyperactivity Disorder (ADHD) in children and adolescents (12-14). Antidepressant effects of Zn have also been reported in a number of experimental and some clinical studies (15, 16). Moreover, effects of adjuvant Zn therapy in the treatment of Obsessive-Compulsive Disorder (OCD) have been reported as well (6). Zinc is a cofactor for the metabolism of different neurotransmitters (13). There is growing the body of evidence implicating over activation of N-Methyl-D-aspartate (NMDA) receptors in the pathophysiology of schizophrenia (17, 18). Moreover, Zn can modulate fast excitatory transmission by a number of mechanisms (19), suppress the increase in extracellular glutamate (19), and inhibit NMDA receptors (16). It can also facilitate the release of Gamma-Amino Butyric Acid (GABA) (6, 19, 20). Evidences of GABAergic involvement in the pathophysiology of schizophrenia have also been discussed in several studies (21-23). Furthermore, it is known that Zn potentiates nicotinic acetylcholine receptors (24). There are also many findings implying cholinergic dysfunction in schizophrenia (25, 26).
In one study, the concentration of Zn in scalp hair of patients with schizophrenia was significantly lower than that in healthy volunteers (27). Reduced serum Zn levels have also been reported in patients with schizophrenia (28). Zinc supplements are commonly used to alleviate a number of conditions, including Zn deficient states, such as diarrhea, age-related macular degeneration, and wound healing (5). To the best of our knowledge, thus far, the efficacy of Zn in the treatment of schizophrenia has not been investigated in clinical trials.
2. Objectives
The purpose of the present study was to assess the efficacy of Zn sulfate as an adjuvant therapy in the treatment of schizophrenia in a 6-week, double-blind, and placebo-controlled trial.
3. Patients and Methods
3.1. Trial Design
This randomized, double-blind, placebo-controlled trial was undertaken in Zare Psychiatric Hospital affiliated to the Mazandaran University of Medical Sciences in Sari City, Iran. This trial is registered with the Iranian Clinical Trials Registry (IRCT registration number: 138801241457N3). The trial was performed in accordance with the ethical standards laid down in the 1995 Declaration of Helsinki (as revised in Edinburgh 2000). All patients and their legally authorized representatives were informed that they could withdraw from the experiment at any time. All of them signed an informed consent form prior to their inclusion in the study. The protocol was approved by the appropriate Ethics Committee on human experimentation of the Mazandaran University of Medical Sciences.
3.2. Participants
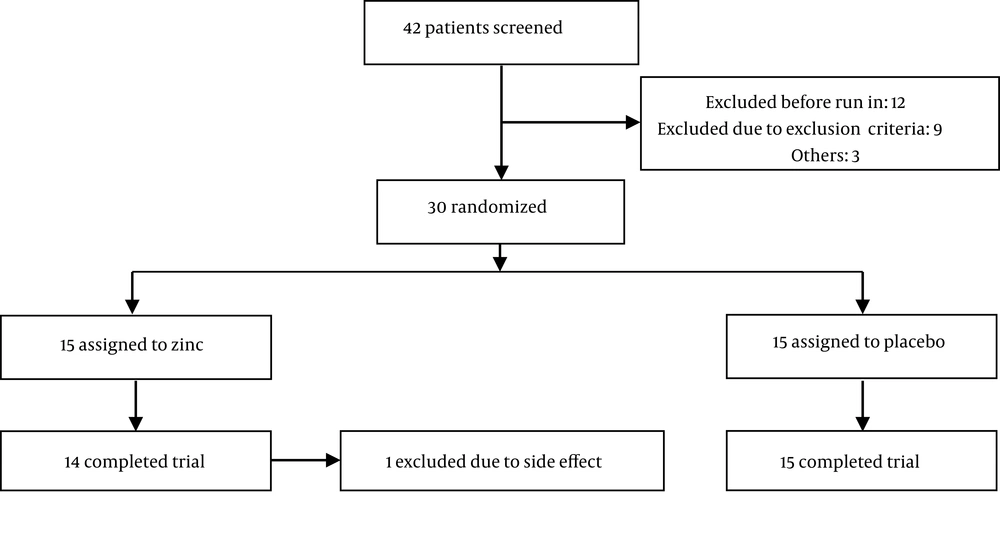
All participants were inpatients, in the active phase of the disorder, who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for schizophrenia. All inpatients were considered as potential study population. A minimum score of 80 on the Positive and Negative Syndrome Scale (PANSS) was required for entering the study. Complete blood count, liver enzymes, and creatinine tests, plus electrocardiography were undertaken at baseline. In addition, a urine test was performed to detect morphine and/or cannabis in urine samples of the patients at baseline. The exclusion criteria included any severe medical conditions (e.g. cardiovascular, renal, hepatic, pulmonary, metabolic, endocrine diseases, or immune system disorders), active peptic ulcer, any clinically significant neurological disorder, current or previous history of psychiatric disorders other than schizophrenia, current substance use or history of substance dependency, history of allergy to Zn or multidrug reaction, taking any medication during the study (except for risperidone, trihexyphenidyl, and lorazepam). Pregnant or lactating women and those of reproductive age without using at least one medically accepted mean of birth control were also excluded. A total of 42 patients were recruited during their hospital stay. Three of them were not eager to enter the trial, and nine other patients excluded due to different conditions including four with the history of drug dependency, two due to abnormal liver enzyme levels, one due to cardiac arrhythmias, one having blood in the urine sample, and another patient who had skin sensitivity. Thirty of the screened patients (male/female = 28/2, age range of 18 - 65 year old) who met the inclusion criteria and did not meet the exclusion criteria of the trial entered the study.
3.3. Interventions
The patients did not receive antipsychotics from a week prior to entering the trial or depot antipsychotics at least 2 months before the study. Risperidone (produced by Sobhan Pharmaceutical Company) was administered in all patients as their standard antipsychotic treatment. It was started with 1 mg twice daily and titrated up to 6 mg/day in all patients during the week. Participants were randomly allocated into two equal groups (n = 15); to one group received risperidone 6 mg/day plus capsules of Zn sulfate 220 mg (each containing 50 mg elemental Zn) three times a day and another group received risperidone 6 mg/day plus identical placebo capsules three times a day for 6 weeks. Placebo capsules were prepared in the pharmaceutical sciences laboratory of pharmacy school, Mazandaran University of Medical Sciences. Patients also received trihexyphenidyl if they faced extrapyramidal symptoms and they received lorazepam in case of agitation or insomnia.
3.4. Outcomes
The PANSS was applied to assess the psychotic symptoms at baseline and week 2, 4, and 6 of the study by a trained third-year resident of psychiatry. The PANSS is a common scale in the assessment of schizophrenia including 30 items, which measures both positive and negative symptoms, as well as general psychopathology by means of a semistructured patient interview. Intensity of each item will be identified by scoring from 1 to 7 in a manner that more severe symptoms get higher scores (29). The rater used standardized instructions in the use of PANSS. The PANSS supplemental aggression risk subscale was also used to evaluate the aggression risk in patients at the same times that the PANSS was applied (30). The mean decrease in PANSS score from baseline was used as the main outcome measure of response of schizophrenia to treatment. There was no significant difference in psychotic symptoms of two groups of patients according to the PANSS scores at baseline (P > 0.05).
3.5. Safety
We were aware of Zn sulfate possible side effects and were looking for them in patients. So far, the only side-effect of Zn sulfate that has been reported to occur significantly more than placebo is a metallic taste (14, 31). Gastrointestinal discomfort (nausea, vomiting, abdominal pain, and diarrhea) has also been reported with the same rate as placebo (14, 31). Skin dermatitis caused by the Zn element of dental fillings has been reported before (32). In the present study, side-effects reported either by the patients or the nurses in the psychiatric ward were recorded. Only one patient in Zn sulfate group withdrew from the trial due to generalized maculopapular reaction.
3.6. Randomization, Allocation Concealment, and Blinding
The stratified block randomization technique was used in our investigation. Eligible participants were stratified based on age (< 25, 25 - 45, or ≥ 45 years old), gender (male, female), and subtype of schizophrenia (paranoid or nonparanoid). Then, by 1:1 ratio using a computer generated code the patients were assigned to receive either Zn sulfate or placebo. The assignments were kept in sealed opaque envelopes until data analysis. Throughout the study, a person who administrated the medications, the PANSS rater, and the patients were all blind to the assignments.
3.7. Statistical Analysis
For descriptive analysis of data, statistical indicators like mean and standard deviation (SD) were used, and the repeated measure Analysis of Variance (ANOVA) (time-treatment interaction) was used to assess the effects of treatment. Two groups were considered as the between-subjects factor and four measurements during treatment were considered as the within-subjects factor (time). This was done for positive, negative, general psychopathology subscale, total PANSS, and supplemental aggression risk scores. GraphPad Prism software version 5 (GraphPad Software Inc., California, USA) was used to analyze, graph and present scientific data. Differences with P < 0.05 were considered as statistically significant.
4. Results
4.1. Patient Disposition and Characteristics
From a total of 42 patients screened for the study, 30 cases were randomly allocated into two groups for trial medication (15 patients in each group) (Figure 1).
No significant difference was found between patients randomly assigned to Zn or placebo group regarding basic demographic data including age, gender, marital status, level of education, and subgroup of schizophrenia (Table 1).
| Variables | Zinc Group | Placebo Group | P Value |
|---|---|---|---|
| 1.00 | |||
| Male | 14 | 14 | |
| Female | 01 | 01 | |
| 0.80 | |||
| < 25 | 05 | 04 | |
| 24 - 45 | 09 | 09 | |
| > 45 | 01 | 02 | |
| 0.90 | |||
| Married | 04 | 04 | |
| Divorced | 03 | 04 | |
| Single | 08 | 07 | |
| 0.59 | |||
| Primary | 10 | 11 | |
| High school | 04 | 02 | |
| Higher | 01 | 02 | |
| 1.00 | |||
| Paranoid | 01 | 01 | |
| Nonparanoid | 14 | 14 |
4.2. Outcomes
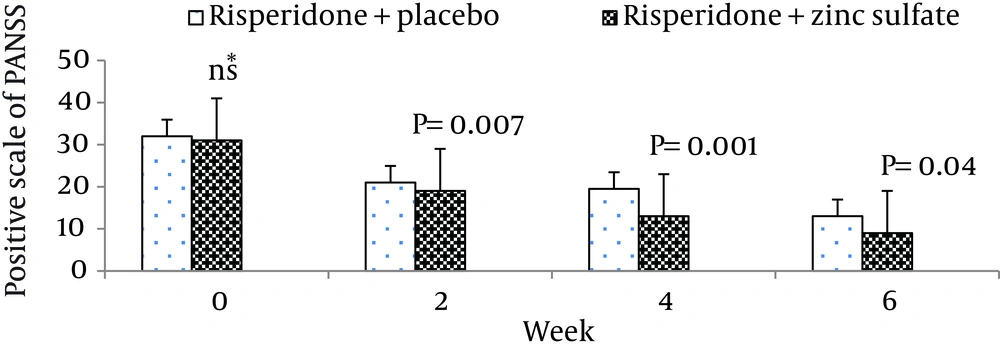
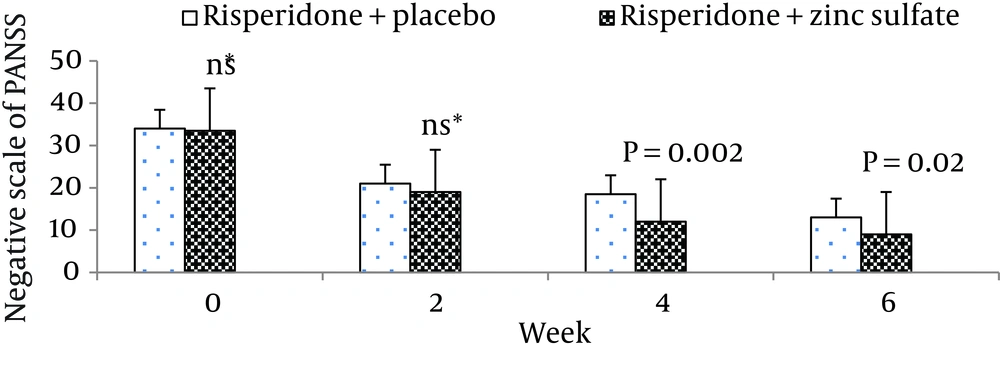
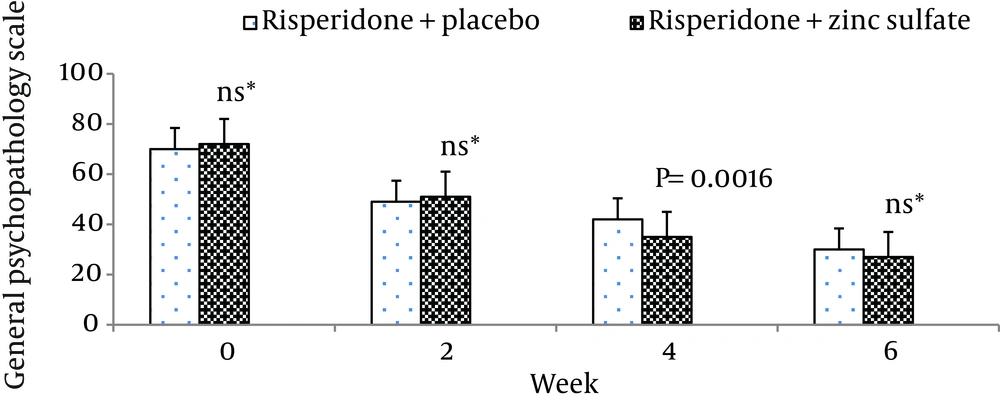
The mean ± standard deviation of positive symptoms scale, negative symptoms scale, the general psychopathology scale, total PANSS score, and the supplemental aggression risk subscale of two groups of patients are shown in Table 2. There were no significant differences between the two groups at week 0 (baseline) on positive symptoms on PANSS (P = 0.55), negative symptoms on PANSS (P = 0.62), general psychopathology on PANSS (P = 0.69), and PANSS total score (P = 0.48).
| Week 0 | Week 2 | Week 4 | Week 6 | |
|---|---|---|---|---|
| Risperidone + zinc sulfate | 31.27 ± 3.84 | 18.13 ± 2.35 | 12.93 ± 2.21 | 09.86 ± 2.82 |
| Risperidone + placebo | 32.13 ± 4.10 | 21.20 ± 3.38 | 18.80 ± 4.24 | 12.87 ± 4.22 |
| Risperidone + zinc sulfate | 32.87 ± 3.29 | 18.67 ± 1.54 | 12.8 ± 2.04 | 9.93 ± 2.21 |
| Risperidone + placebo | 33.67 ± 5.26 | 20.67 ± 3.67 | 18.2 ± 4.32 | 13.00 ± 4.50 |
| Risperidone + zinc sulfate | 70.20 ± 6.13 | 46.40 ± 3.71 | 33.40 ± 4.12 | 24.53 ± 5.63 |
| Risperidone + placebo | 69.47 ± 3.50 | 45.20 ± 4.45 | 40.33 ± 6.46 | 27.60 ± 6.55 |
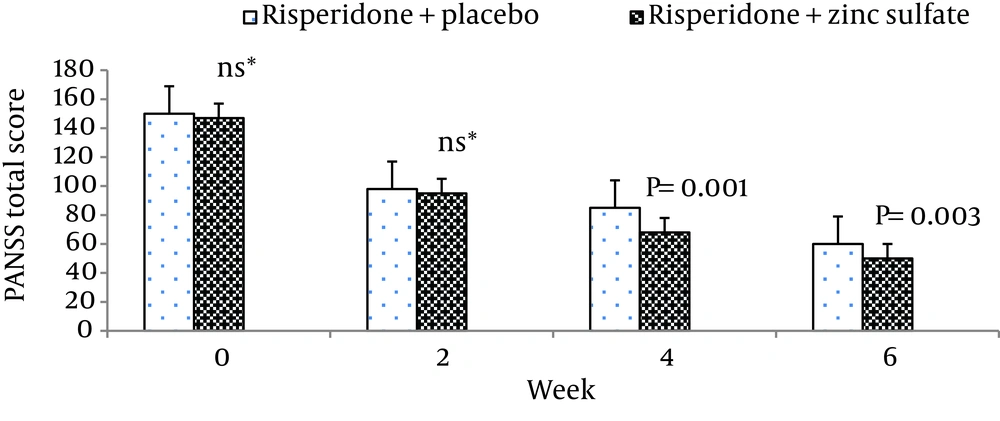
| Risperidone + zinc sulfate | 147.50 ± 11.72 | 91.67 ± 6.76 | 66.00 ± 7.07 | 49.80 ± 12.15 |
| Risperidone + placebo | 149.90 ± 6.14 | 96.60 ± 8.38 | 85.73 ± 13.00 | 60.60 ± 14.16 |
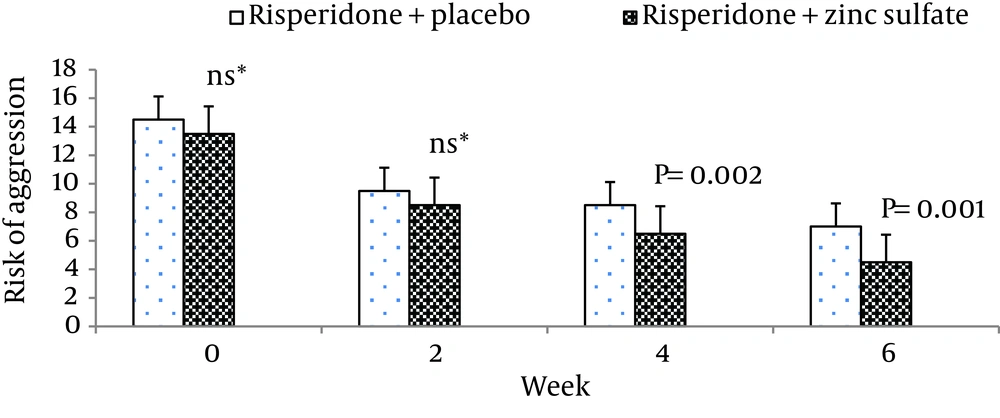
| Risperidone + zinc sulfate | 13.30 ± 3.22 | 8.60 ± 1.92 | 06.86 ± 1.30 | 04.80 ± 1.60 |
| Risperidone + placebo | 14.60 ± 1.63 | 9.53 ± 1.40 | 08.53 ± 1.40 | 07.33 ± 1.23 |
a Data are presented as mean ± SD.
b Positive and negative syndrome scale.
Moreover, there was no significant difference between the two groups at baseline regarding risk of aggression (P = 0.12). The differences between the two groups on the positive scale, negative scale, the general psychopathology scale, total PANSS score, and the PANSS supplemental aggression risk subscale scores are demonstrated in Figures 2 - 6, respectively.
5. Discussion
Schizophrenia is estimated to be the 8th leading cause of disability-adjusted life years worldwide in the age group 15 - 44 years (1). It is characterized by a diverse range of symptoms, including positive symptoms (such as hallucinations and delusions), negative symptoms (such as social withdrawal and diminished affective responsiveness), and cognitive deficits (33). Its treatment involves a combination of psychosocial rehabilitation and pharmacotherapy (3). Despite the improvement of antipsychotics, treatment of schizophrenia is still a big challenge for clinicians (2, 3). Thus, several studies are ongoing for identifying drugs of different classes, other than dopamine antagonists, that may enhance the response to antipsychotics, or that may even have efficacy as monotherapy (2, 17, 34).
The results obtained in the present trial demonstrate that adding Zn to an atypical antipsychotic regimen is effective in the improvement of symptoms of schizophrenia and risk of aggression in patients with schizophrenia. Both studied groups, treated with risperidone during the 6-week trial, showed a significant improvement on PANSS total score and on its all subscales as well as the supplemental aggression risk subscale. The Zn group had significantly greater improvement in total score of PANSS, positive and negative symptoms, and risk of aggression over the 6-week trial. Baseline characteristics of patients including sex, age, marital status, level of education, and subgroup of schizophrenia did not differ between the groups; thus, cannot explain differences in the therapeutic outcome. To the best of our knowledge, this study was the first double-blind and placebo-controlled clinical trial investigating the efficacy of Zn in the treatment of schizophrenia. Complementary medicines, such as trace element supplements, can be assumed as proper candidates for investigation in chronic psychiatric disorders due to lower risk of side-effects as well as better acceptance among people (35). Zinc is an essential trace element in the body (6, 7), which is required for development of the brain and also for its function (9-11). So far, positive effects of supplemental Zn on depression (15, 36, 37), OCD (6), ADHD (14, 31), and AD (11) have been reported in other studies.
To the best of our knowledge, there is no report of kinetic interactions between Zn sulfate and risperidone. This leads us to assume that the therapeutic effect observed by Zn sulfate on symptoms of schizophrenia is likely to result from a pharmacodynamic mechanism. It has been reported that Zn serves as an endogenous neuromodulator of several important transmitters (19) and this role can be used for explaining its mechanism of action in the improvement of schizophrenia. There is growing the body of evidence implicating glutamatergic system in the pathophysiology of schizophrenia (4, 17, 18, 38). Along with the studies reporting the efficacy of NMDA receptor allosteric agonists at the glycinB site in schizophrenia (4) some scientists have hypothesized the possible efficacy of NMDA antagonists in this disorder (4, 39). A preliminary study has even shown the probable efficacy of memantine as a noncompetitive NMDA antagonist in improvement of schizophrenia (39). Also, Zn can modulate fast excitatory transmission by a number of mechanisms (19). It suppresses the increase in extracellular glutamate (19) and inhibits NMDA receptors (16). An ex-vivo study has suggested that low-affinity NMDA receptor antagonists might even interact with dopamine receptors by binding the dopamine transporters (40). Evidences of GABAergic involvement in the pathophysiology of schizophrenia have also been discussed in several studies (22, 23). However, a recent meta-analysis did not find significant therapeutic benefits in addition of benzodiazepines to the antipsychotic regimen of schizophrenic patients (41). In a small preliminary randomized trial, selective benzodiazepine acting at the GABAa receptor improved working memory in patients with schizophrenia (22). Although a similar investigation in a greater sample size showed only little benefit of that prodrug, this receptor remains a promising target (42). Moreover, Zn facilitates the release of GABA (6, 19, 20) and may improve symptoms of schizophrenia via this mechanism.
It is known that Zn potentiates nicotinic acetylcholine receptors (24). There are also many findings implying cholinergic dysfunction in schizophrenia (25, 26, 43). In two preliminary studies, a cholinergic nicotinic partial agonist (25) and muscarinic cholinergic agonist (43) showed some efficacy in improving symptom scores and cognition in patients with schizophrenia, which the results are in line with ours.
The impact of Zn on improvement of negative symptoms of schizophrenia can also be due to its antidepressant implications, which have been reported in previous studies (15, 36, 37). Zinc also has antioxidant properties (5, 8), which can be considered as another explanation for its effectiveness in schizophrenia. There are studies reporting increased production of reactive oxygen or decreased antioxidant protection in patients with schizophrenia that strengthen the hypothesis suggesting the role of excessive free radical production or oxidative stress in the pathophysiology of this disorder (33).
In one study, the concentration of Zn in scalp hair of schizophrenic patients was significantly lower than that in healthy volunteers (37). Reduced serum Zn levels have also been reported in patients with schizophrenia (28). However, the extracellular Zn concentration may not be a good indicator of Zn concentration in the body (19).
In our study, adding Zn to risperidone was effective in reducing aggression. A study demonstrated that mean plasma Zn values were significantly lower in criminal schizophrenic men compared with noncriminal subjects (44). Another study had also reported that blood copper/Zn ratios in assaultive young males were high when compared to a control group of young males with no history of an assaultive behavior (45).
Administration of 220 mg of Zn sulfate three times a day was well-tolerated, and no major clinical side-effects were detected. However, due to the lack of systematic recording of side-effects during the trial, which should be considered as a shortcoming of this study, there may have been some missed side-effects that were neither reported by our patients nor observed by the nurses. Other limitations of the present study include using only one dose of Zn sulfate, the small number of participants, short period of follow-up, and lack of a plasma Zn concentration that all indicate the need for future studies. In line with our hypothesis, adding Zn to an atypical antipsychotic was particularly effective in the improvement of positive and negative symptoms subscales on PANSS, general psychopathology subscale, and total score of PANSS as well as risk of aggression in patients with schizophrenia.