1. Background
Consumption of stimulants such as cocaine and amphetamines is a serious social concern that has caused significant health problems. According to global statistics, the number of methamphetamine users is estimated as 35 million (1). Methamphetamine-use disorder (Meth-use disorder) has high rates of relapse. A report found that about 61% of meth users, who received treatment for methamphetamine dependence, returned to active methamphetamine use within one year after treatment (2). Furthermore, at least some biological damages caused by methamphetamine seem to be permanent and irreversible (3). Therefore, reducing relapse is the most important aspect of therapeutic strategies to treat Meth-use disorder. It worth noting that relapse still is a major challenge in drug therapy (4).
Although little information is available about the underlying etiological, behavioral, and neurobiological mechanisms of this problem, it’s established that the rewarding effect of meth plays a pivotal role. There are evidence indicating that dopaminergic stimulation in the mesocorticolimbic system, especially in the nucleus accumbens, is responsible for methamphetamine and cocaine rewards phenomena (5). Methamphetamine-induced damage not only degenerates dopaminergic (DA) nerve terminals, but also reduces dopamine transporter (DAT) activity, tyrosine hydroxylase activity, and vesicular monoamine transporter-2 (VMAT) protein levels (6, 7). Decreased concentrations of DA and DA metabolites have been shown in several brain regions of rodents or nonhuman primates following repeated administration of methamphetamine (8, 9).
There are several lines of evidence suggesting that this pathway is impacted by the inhibitory effect of the GABAergic system, especially GABAA. They have shown that benzodiazepines, through influencing the GABAA receptor, can impress the release and turnover of dopamine in the mesocorticolimbic pathway. Cellular methods of these bidirectional influences have been investigated in previous studies (10-13).
Although, the effect of benzodiazepine and other GABAergic factors have been assessed in several behavioral studies in the context of Meth-use disorder (5, 14-21), however, the available evidence is heterogeneous and inconsistent. Some studies have suggested the positive effect of diazepam, oxazepam, alprazolam, clonazepam, and other benzodiazepines in attenuating drug-seeking behaviors in the conditioning and extinction phase (5, 15, 17-19, 22, 23). On the other hand, there are research that reported contradicting findings (23, 24).
Moreover, few studies have examined the benzodiazepine effect in relapse and reinstatement period (22). There are various methods to evaluate the behavioral effect of drugs and substances and reward system activity. Two most prevalent methods are drug self-administration and conditioned place preference (CPP) paradigm. The first is based on operant conditioning procedure and the second follows classical conditioning (25).
The CPP has been widely used to evaluate the effects of methamphetamine on reward properties in rodents. Moreover, it has been indicated that preference for the drug-paired environment can be extinguished and subsequently reinstated by either drug priming or stressors (25).
The current study aimed to evaluate the possible effects of diazepam administration during and after the withdrawal period on the Meth-seeking behavior using CPP after exposure to a single dose of methamphetamine.
2. Objectives
The current study aimed to investigate the effect of diazepam administration during and after the withdrawal period on methamphetamine-seeking behavior of conditioned place preference in male Wistar rats.
3. Methods
In this animal study, 24 male Wistar rats (weighing 200 - 250 g) that were divided into three groups are studied. The sample size was calculated according to previous similar studies (26). All rats were purchased from the Pasteur Institute. The subjects were kept in a research animal room at 22°C and a 12-hour dark-light cycle with adequate water and food, plus one week for acclimatization.
3.1. The Test Groups of This Research Are as Follows
3.1.1. Control Group
This group, which contained two subsequent groups, received intraperitoneal methamphetamine and saline administration in the conditioning phase with 6-hour intervals; the rats in the extinction phase and the priming step, received saline and 0.5 mg/kg methamphetamine, respectively.
3.1.2. The First Group
This group received intraperitoneal methamphetamine and saline in the conditioning phase as well as 3 mg/kg diazepam (daily) in the extinction phase and 0.5 mg methamphetamine in the priming step.
3.1.3. The Second Group
For this group, the conditioning phase was performed like the previous group (injection of methamphetamine and saline with a 6-hours interval). Rats received an intraperitoneal injection of saline at the extinction phase and 15 minutes before the priming phase received a single dose of 3 mg/kg diazepam. Besides, 0.5 mg/kg methamphetamine was injected in the priming phase.
Characteristic of the three groups is described in Table 1.
| Group | Conditioning Phase | Extinction Phase | Priming Phase | ||
|---|---|---|---|---|---|
| Pre | Con | Post | |||
| Control | - | Saline/Meth | - | Saline | Meth 0.5 mg/kg |
| Group 1 | - | Saline/Meth | - | Diazepam 3 mg/kg | Meth 0.5 mg/kg |
| Group 2 | - | Saline/Meth | - | Saline | Diazepam 3 mg/kg + Meth 0.5 mg/kg |
Interventions Provided for the Three Groups Separated by Experiment Phase
Initially, the rats were trained by the CPP. So that a box with three quadrangles was used. two side quadrangle were the same size (30 × 30 × 30 cm), and they only differed in their visual and tactile signs used, and a small central quadrangle, which had a way to two other chambers. The link between the central chambers with the other two chambers was made using a sliding door. A camcorder was placed on the top of the box, which was used to continuously monitor and record the behavior of the rats. At the end of the experiment, the recorded behavior was examined using the tracking software.
3.1.4. Different Stages of the Experiment
Before providing each intervention, the rats were weighed. They had 15 to 20 minutes time to adapt themselves to the laboratory environment. Then, the behavioral stages of the research were done as follows:
3.1.4.1. Preconditioning
On the first day, in order to create a place preference, the preconditioning stage was performed. To familiarize the rats with the environment, they were first placed in the central chamber for about one minute, while the sliding doors were closed. Then, the doors were opened and the rats were allowed to go freely within all three chambers for ten minutes. The time spent in each chamber and the rats’ motor activity were measured. Afterward, they were taken back to their cage. In order to remove any initial bias of the rats towards each chamber, the spent time in the central chamber was reduced from the total of fifteen minutes, and the remainder was divided into two parts. The resulting number was equal to the optimal time for each rat to pass without bias in each chamber. Then this optimal time was multiplied by 0.3 and the obtained value, once, was summed up with optimal time and it was reduced. By doing so, there would be an interval that if a rat spent a period of time out of it, in a particular chamber on the first day, it was assumed that there was a bias for that chamber and the rat was excluded. The remaining rats were randomly distributed among the study groups.
3.1.4.2. Conditioning
On the second, third, and fourth days of the experiment, the stage of conditioning was performed. On each of these days, the rats received methamphetamine (1 mg/kg) and then were placed in one of the chambers (vertical striped floor) for a period of 30 minutes, while the sliding door was closed. Then, the rats were taken back to their cages, and after 6 hours they were restrained for another 30 minutes on the other chamber (the horizontal striped floor), while they received saline. For rats of each group, chambers that were associated with the drug or saline and the order of taking the drug or saline was distributed equally. Therefore, each rat learned that one of the lateral chambers is related to the rewarding drug and the other is not.
3.1.4.3. Post-Conditioning
On the fifth day, the CPP test was performed. Initially, rats were placed in the central chamber for one minute. Then, doors were opened and the rats were allowed to go freely within the chambers for ten minutes. During this time, the duration that the rats spent in each chamber and their motor activities were measured and recorded. The place of preference was defined as the difference between the time spent on the drug-related chamber on the test day and the spent time in the same chamber on the first day. The rats in group 1 received diazepam (3 mg/kg/daily) during the extinction period. Afterward, on the seventeenth day, after triggering the drug-seeking behavior, they were tested by CPP. To stimulate the drug-seeking behavior, a single dose of methamphetamine (0.5 mg/kg intraperitoneal) was injected.
It should be noted that the eleven-day course to withdraw methamphetamine is only an estimation based on previous studies. Hence, we also tried to investigate this assumption in the present study. Therefore, from the beginning, the CPP test was daily performed to clarify the time of complete withdrawal.
3.1.5. Ethical Considerations
In the present study, all procedures were approved by the ethical committee of Tehran University of Medical Sciences and were consistent with the internationally accepted principles for the Care and Use of Laboratory Animals as found in the US guidelines (NIH publication no.: 80-23, revised in 1996). Rats were kept in groups of 6 in the animal house of the neuroscience laboratory of Tehran University of Medical Sciences with a controlled temperature of 23 ± 2°C, regular 12-hour light/dark cycles (light on 07:00 - 19:00). Food and water were provided ad libitum. To reduce stress induced by the laboratory environment and CPP apparatus, the animals were brought to the lab the day before the experiment and were placed in the CPP box for 30 minutes. The tests were performed at the same time of the day (8:00 and 15:00) to reduce circadian rhythm effects.
3.2. Statistical Analysis
All data were plotted and analyzed using Graph Pad Prism 6 software. Data are presented as mean ± SEM. Statistical significance was assessed using ANOVA with the Student Newman-Keuls complement test. Paired samples t-test was used to compare the CPP score during the post-conditioning test and the pre-conditioning test. Statistical significance was considered when P-value < 0.05.
4. Results
4.1. Periods of Acquisition, Extinction, and Reinstatement of Conditioned Place Preference in the Control Group
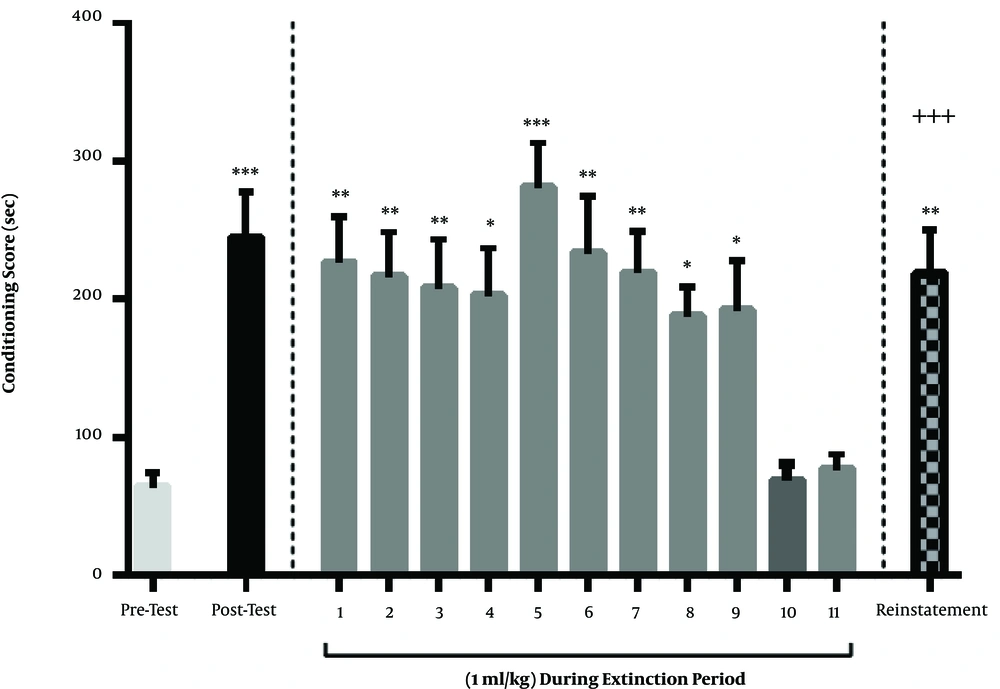
Methamphetamine at a dose of 1 mg/kg induced a CPP. Based on the findings, a 3-days injection of 1 mg/kg of methamphetamine caused a significant bias toward the chamber that was associated with methamphetamine. A three-day injection of this dose of methamphetamine, based on the CPP protocol, caused a significant bias toward the chamber that was associated with methamphetamine, the day after conditioning. Therefore, there was a significant difference in CPP scores between the day before and after the conditioning (P < 0.001). This group of rats received saline, instead of diazepam, during the period of extinction and were tested for CPP each day. The findings related to this group showed that the bias toward the chamber associated with methamphetamine or, in other words, the conditioning score, gradually decreased during the days after the conditioning; finally, on the 10th and 11th days of the extinction period, there was no significant difference between the conditioning score with pre-conditioning day (P > 0.05). These results showed that the extinction period lasted until the 10th or 11th days in the control group. One day after the last day of the extinction (12th day), the rats were tested for drug-seeking behavior. A single-dose of ineffective methamphetamine (0.5 mg/kg) was injected. They were immediately placed in the CPP box and their conditioning score was calculated. The results showed that this dose of methamphetamine could induce a significant drug use relapse. On this day, there was a significant difference between the conditioning score of reinstatement day and the last day of extinction. Also, there was a significant difference between the conditioning score of reinstatement day and post-conditioning day. In other words, in this day, just like the day after the conditioning, the rats showed a significant bias toward the chamber that was associated with methamphetamine (Figure 1).
Periods of acquisition, extinction, and reinstatement of methamphetamine-induced CPP in the control group. Three days of methamphetamine injection of 1 mg/kg resulted in a positive conditioning score on the day after conditioning. During the extinction period, the rats were injected with saline (1 mL/kg) each day. Extinction occurred on the 10th and 11th days of the extinction period. On the day of reinstatement, the ineffective single dose of methamphetamine (0.5 mg/kg) resulted in relapse and an increase in the conditioning score of animals. Each column represents the mean ± SEM of eight rats. *, P < 0.05; **, P < 0.01 and ***, P < 0.001 compared with pre-test day. +++, P < 0.001 compared with the last day of the extinction period.
4.2. The Effect of Diazepam Injection During the Extinction Period on Maintenance and Reinstatement of Methamphetamine-Induced CPP
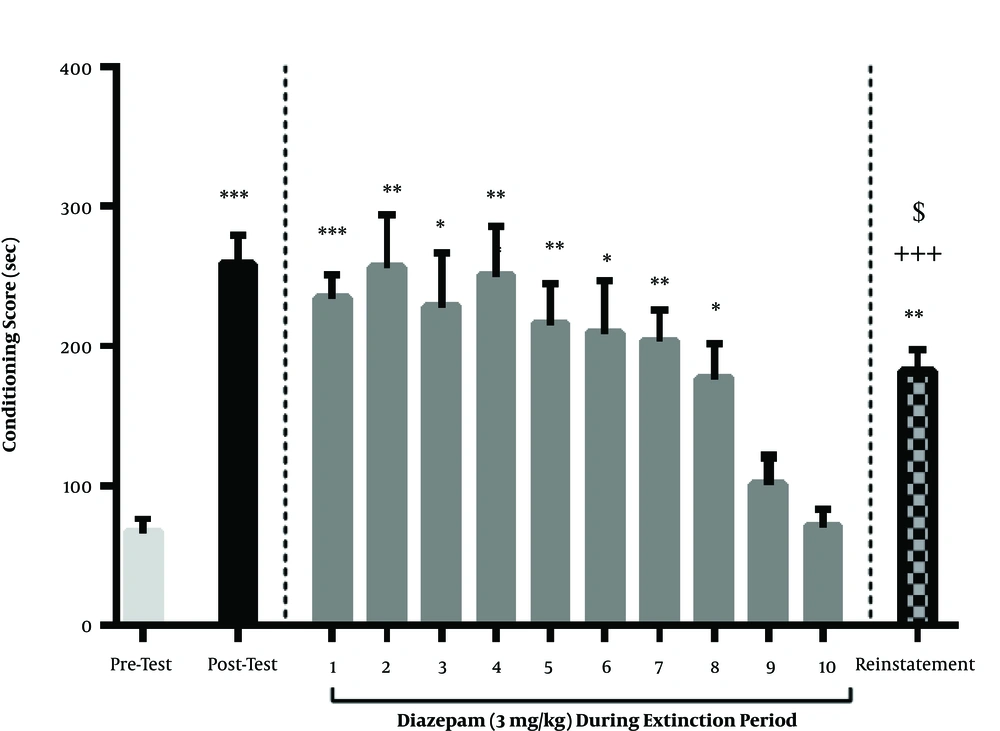
In this section, the effect of diazepam injection (3 mg/kg) on the extinction and reinstatement of methamphetamine was investigated. According to the Newman-Keuls complement test [P < 0.0001], we could use the one-way analysis of variance. The results showed that extinguished preference for the Meth-paired compartment occurred one day earlier in the animals treated with this dose of diazepam compared to the control group. One day after the extinction phase, a priming dose of methamphetamine was injected 30 min before the final test for CPP. Although on this day the CPP score was greater than pre-test day, but there was a significant difference concerning the CPP score between reinstatement and post-test day, indicating that the reinstatement of the place of preference primed by methamphetamine injection was suppressed by diazepam treatment during the extinction period (Figure 2).
The effect of diazepam administration (3 mg/kg) during the extinction period on the maintenance and reinstatement of methamphetamine-induced CPP. Diazepam injection (3 mg/kg/daily) during the extinction period reduced the extinction season by 1 day and extinction occurred on the 9th and 10th day. Also, this treatment was associated with a significant decline in reinstatement conditioning scores compared to the post-test phase. Each column represents the mean ± SEM of eight rats. **, P < 0.01 and ***, P < 0.001 compared to the pre-test day. +++, P < 0.001 compared with the last day of the extinction period. $$, P < 0.001 compared with the post-test day.
4.3. The Effect of Single-Dose of Diazepam Before Priming on the Reinstatement of Methamphetamine-Induced CPP
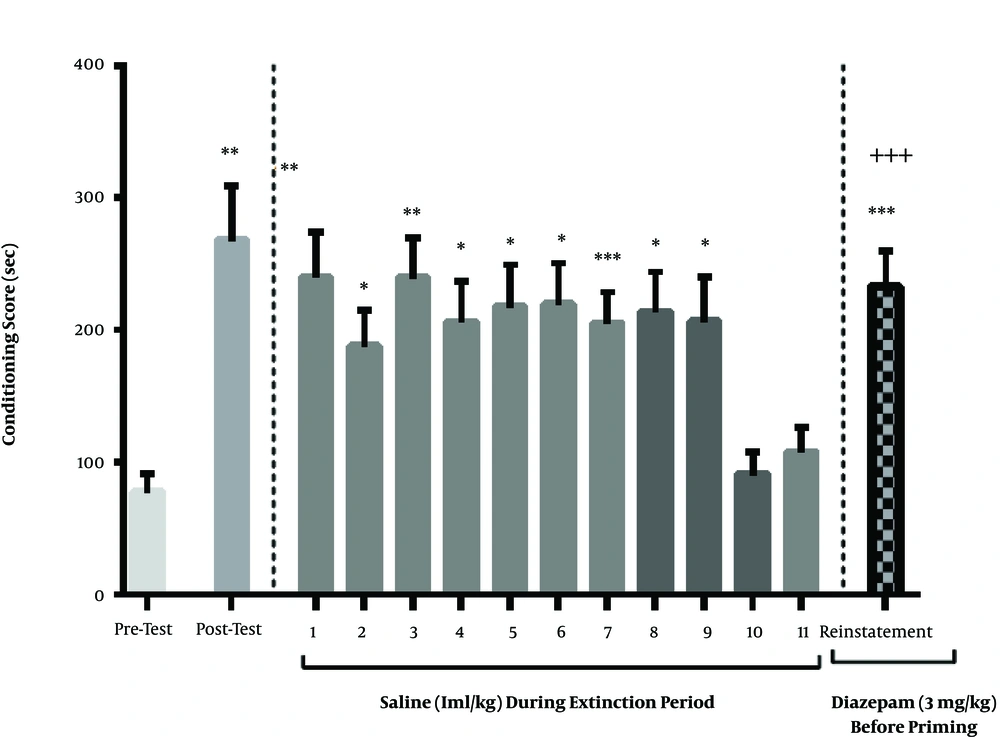
The results of this section indicated that diazepam injection (3 mg/kg) before the priming dose of methamphetamine had no significant effect on the relapse (P > 0.05). As shown in Figure 3, the extinction occurred on the 10th or 11th day. Moreover, the ineffective dose of methamphetamine increased the conditioning score like the day after the conditioning, and there was no significant difference between them (Figure 3).
The effect of single-dose of diazepam before priming on the reinstatement of methamphetamine-induced CPP. The diazepam injection (3 mg/kg) before priming did not affect the reinstatement conditioning score compared to the post-test phase. Each column represents the mean ± SEM of eight rats. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 compared with pre-test day. ++, P < 0.01 compared with the last day of the extinction period.
5. Discussion
This study aimed to evaluate the effect of diazepam administration during and after the withdrawal period on the drug-seeking behavior induced by the single-dose methamphetamine injection in previously methamphetamine-dependent male rats that were abstinent during the study. We used a CPP paradigm, which is a classic model for assessing the effects of drug-related rewards, in order to evaluate the mechanisms through which methamphetamine yields rewarding memories (25). Based on the findings, a 3-days injection of methamphetamine (1 mg/kg) caused a significant bias toward the chamber that was associated with methamphetamine. This finding is consistent with the results reported by previous studies that utilized a similar dose of methamphetamine (14, 27-29).
Recent evidence demonstrated that dopamine is the main factor for normal ‘wanting’, and its activation in the nucleus acumens can be sufficient to enhance cue-triggered compulsive ‘wanting’ in addiction (30). There are evidence indicating that psychostimulants including methamphetamine, increases dopamine release in this brain area (5, 30). Therefore, increasing the CPP score by administering methamphetamine can be expected. In the present study, administering diazepam (3 mg/kg) reduced the extinction period shortly, and the significant reduction in the withdrawal period occurred on day one and for the extinction period, it happened on the 9th and 10th days. This effect showed that daily administration of diazepam leads to a faster attenuation of CPP score in rats. Our data also showed that the reinstatement of the place of preference primed by the methamphetamine injection was suppressed by diazepam treatment during the extinction period.
Previous studies evaluated the effect of benzodiazepines and other GABA agonists in CPP induced with psychostimulants in both conditioning and extinction periods. For instance, a study reported that oxazepam (benzodiazepine agonist) administration before the final methamphetamine CPP test in methamphetamine-dependent rats led to significant declines in CPP. In the same study, oxazepam (10 mg/kg ip) was injected 30 minutes before CPP testing following 4 days of conditioning (15). Leri et al. (17) demonstrated that injecting diazepam before conditioning with methamphetamine and its co-administration in this phase attenuated the CPP induced with methamphetamine. This effect was not observed in morphine conditioned rats (17).
In another research, Meririnne et al. (5) compared the effect of diazepam and zolpidem administration on amphetamine and cocaine-induced CPP in rats. The conditioning phase in their study lasted for 8 days. They injected diazepam and zolpidem to separated groups during the conditional periods. For both methamphetamine and cocaine conditioned rats, receiving diazepam was associated with reduced CPP in post conditional phase, but zolpidem was less effective. They concluded that differential distribution of omega1- and omega2- receptors in the brain is responsible for such observation (5). On the other hand, their results are not consistent with the findings reported by Spense et al. (19), which reported that oxazepam attenuated methamphetamine self-administration but alprazolam did not have such effect. To figure out if this result arose from the GABAA receptor or translocator protein (TLPO), they used antagonists of GABAA and TLPO before administration of alprazolam and oxazepam. They concluded that GABAA is responsible for the enhancement of methamphetamine self-administration in the alprazolam group (19), though they measured drug-seeking behavior via drug self-administration that is based on the operant conditioning rule.
Although previous studies have reported conflicting results, it seems that most studies indicated promising results in the case of using benzodiazepines in individuals that recently were dependent on methamphetamine or other psychostimulants. None of the abovementioned studies have evaluated the effect of benzodiazepine on the reinstatement phase. In fact, few studies have investigated this issue. Our results showed that a single dose of diazepam before the priming dose of methamphetamine could not prevent the reinstatement of methamphetamine-induced CPP.
Ito et al. (22) also reported findings that are mostly consistent with our results. They indicated that treatment with clonazepam, a benzodiazepine agonist, before each methamphetamine administration during the dependence period could prevent the emergence of behavioral sensitivity to methamphetamine, which occurs after the withdrawal period accompanied by increased locomotor activity in rats. Pretreatment with flumazenil, as a benzodiazepine antagonist, can prevent this effect of clonazepam. In the final part of their research, they administrated clonazepam (1 mg/kg) before the methamphetamine challenge (1 mg/kg). The rats were previously sensitized to methamphetamine (within a 10-day period) and the challenge was performed on day 17 or 18. The results showed motor activity enhancement. They found that clonazepam could not prevent the development of behavioral sensitivity to methamphetamine, after the appearance of this behavior (22). It seems that different mechanisms are responsible for primary sensitization and relapse. There are studies that reported changes in the ventral tegmental area, prefrontal cortex, and ventral pallidum other than nucleus accumbence are responsible for the pattern of expression of behavioral sensitization (31).
Cellular studies have illustrated two types of changes in the somatodendritic dopamine region elicit by exposure to sensitizing drugs or stressors: (1) the dopaminergic cell groups; and (2) GABA regulation of dopaminergic cell groups. These changes cause the dopaminergic neurons of sensitized animals to become either increasingly sensitive to excitatory pharmacological and environmental stimuli or desensitized to inhibitor regulation. These changes in the terminal field can alter patterns of dopamine release in sensitized animals (32).
However, this phenomenon might be a possible mechanism. We know that multifarious mechanisms and neurotransmitters are involved in the long-term potentiation and reward phenomenon. Thus, further studies are needed to further reveal the underlying mechanisms of relapse and therapeutic strategies.
5.1. Conclusions
This study, by providing evidence at the behavioral level, demonstrated that administration of diazepam during extinction period is associated with declined maintenance and reinstatement of Meth-induced CPP. Moreover, the effects of diazepam can to somehow be attributed to dose, duration, and timing of diazepam administration as well as age and gender of the rats. Future studies are needed to extend our knowledge beyond the effects of benzodiazepines on drug taking behavior.