1. Background
Methadone maintenance therapy (MMT) is favorable for the treatment of opioid dependence (1). In Iran, the prevalence of opioid use is rising and was nearly three times higher than the prevalence worldwide. About 1.2 million Iranians have opioid dependency (2). About 500,000 people are under buprenorphine and methadone therapy (3). Drug abuse (i.e., opioids and methadone) can increase inflammatory parameters and oxidative stress (4). In vitro and in vivo medical evidence has reported that opioid dependence has complication immunomodulatory impacts on adaptive immune responses (5) and inflammatory markers (6). Increased gene expression of inflammatory cytokines has been evaluated in noradrenergic locus coeruleus cells in the brains of opioid-dependent patients (7). In addition, it was reported that individuals with heroin dependence encounter energy reduction, carbohydrate and protein consumption, and elevation in fatty acids intakes for four-year MMT (8).
Recently, the issue of vitamin D administration is suggested in patients under MMT. This may be due to the favorable effects of vitamin D in subjects under MMT and diseases associated with metabolic disorders. Previous studies demonstrated that prescription of a pharmacologic dose of vitamin D (50,000 IU/2-week) for 3 months in subjects under MMT improved mental health scales and metabolic outcomes (9). Whether such improvements in biochemical variables would translate into clinically meaningful reductions in the harmful sequelae of patients under MMT remains unknown. Regarding the effects of vitamin D administration on inflammatory cytokines, a study of colorectal cancer subjects demonstrated reduced circulating levels of inflammatory markers after vitamin D intake (10). In subjects under hemodialysis, supplementation with vitamin D significantly decreased inflammatory factors (11); however, the inflammatory cytokines could not be ameliorated in non-diabetic population (12). In a study related to the effect of vitamin D on peroxisome proliferator-activated receptor gamma (PPAR-γ), high-dose vitamin D for 2 months significantly elevated PPAR-γ gene expression (13). In another study, vitamin D deficiency led to the dysregulation of glycemic control in Goto-Kakizaki rats by decreasing gene expression of PPAR-γ (14).
Vitamin D may result in improving mental health symptoms, metabolic profiles, biomarkers of oxidative stress and inflammation through their effect on central nervous system, neuronal circuits and the regulation of pro-inflammatory cytokines in cellular systems (9, 10, 15), as well as affecting signaling pathways and gene expression (16). In addition, vitamin D-treated animals, which consumed methamphetamine demonstrated that the oral taking of vitamin D can decrease metabolites and protection of dopaminergic system against dopamine and serotonin depleting (17). Although the impacts of consumption of vitamin D on psychological outcomes have attracted a great deal of attention, data on the gene expression related to inflammatory cytokines and insulin metabolism in subjects under MMT are limited and contradictory. The current study, therefore, aimed to evaluate the effects of colecalciferol intake on gene expression involved inflammatory cytokines and insulin metabolism in patients under MMT.
2. Objectives
Owing to the importance of vitamin D, this study aimed at examining vitamin D supplementation on withdrawal symptoms and genetic response in patients under MMT.
3. Methods
3.1. Participants
This study was registered in the Iranian website for registration of clinical trials as http://www.irct.ir, no: IRCT2017042433551N1, in accordance with the Declaration of Helsinki guideline. In addition, written informed consent was taken from all participants. Ethical approval code was IR.Kaums.REC.1395.114 for this study. This investigation was conducted on 40 patients undergoing MMT who referred to the Golabchi Clinic in Kashan, Iran. The included subjects were aged 18 - 60 years, currently undergoing MMT and opioid dependence in the past year. Exclusion criteria were using colecalciferol 3 months before the intervention, and people with a history of metabolic diseases, including diabetes, cardiovascular and hypertension before the intervention, and unwillingness to contribute during the intervention.
3.2. Study Design
At baseline, male patients were randomly divided into two groups to receive either 50,000 IU vitamin D (Zahravi Pharmaceutical Company, Tabriz, Iran) or placebo (Barij Essence, Kashan, Iran) (n = 20 each arm) every 2 weeks for 12 weeks. Randomization was performed using computer-generated random numbers. The lack of information about the suitable vitamin D dosage for subjects with MMT made us use the above-considered dose of vitamin D based on a previous report in chronic liver disorder cases (18). Methadone was administered as syrup for the subjects. Compliance with the colecalciferol and placebo intake was done by the quantification of 25 (OH) vitamin D values.
3.3. Clinical Assessment
The clinical opiate withdrawal scale (COWS), as a reliable and valid measure for evaluating withdrawal signs, was assessed to examine withdrawal symptoms at baseline and after a 3-month intervention (19). Nazari et al. (20), approved the validity and reliability of Persian version of COWS among Iranian population with an opiate withdrawal syndrome. In the above-mentioned questionnaire, Cronbach’s alpha coefficients ranged from 0.66 to 0.88, while validity and reliability coefficients were 0.73 and 0.88, respectively.
3.4. Assessment of Outcomes
Interleukin-1 (IL-1), IL-8, and tumor necrosis factor alpha (TNF-α) expression as the primary outcome and PPAR-γ expression and withdrawal symptoms were considered the secondary outcomes. Furthermore, 25-hydroxyvitamin D values were evaluated using an ELISA kit (IDS, Boldon, UK).
3.5. Isolation of Lymphocyte Cells
Fasting samples (10 mL) were collected in Kashan Reference Laboratory at both baseline and endpoint of the intervention. Samples were taken for cell count and viability testing using trypan blue, as well as RNA and DNA extraction (21). Each sample’s OD 260/280 ratio ranged from 1.7 to 2.1, which showed no contamination with both protein and DNA (21). Gene expression of IL-1, IL-8, TNF-α, and PPAR-γ was assessed by quantitative RT-PCR, using LightCycler technology (Roche Diagnostics, Rotkreuz, Switzerland) with SYBR green detection and Amplicon Kit (Table 1).
| Gene | Primer | Product Size, bp | Annealing Temperature, °C |
|---|---|---|---|
| GAPDH | 126 | 61.3 | |
| F | AAGCTCATTTCCTGGTATGACAACG | ||
| R | TCTTCCTCTTGTGCTCTTGCTGG | ||
| IL-1 | 174 | 56 | |
| F | GCTTCTCTCTGGTCCTTGG | ||
| R | AGGGCAGGGTAGAGAAGAG | ||
| IL-8 | 150 | 56 | |
| F | GCAGAGGGTTGTGGAGAAGT | ||
| R | ACCCTACAACAGACCCACAC | ||
| TNF-α | 188 | 52 | |
| F | GTCAACCTCCTCTCTGCCAT | ||
| R | CCAAAGTAGACCTGCCCAGA | ||
| PPAR-γ | 210 | 54 | |
| F | ATGACAGACCTCAGACAGATTG | ||
| R | AATGTTGGCAGTGGCTCAG |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-1, interleukin-1; IL-8, interleukin-8; PPAR-γ, peroxisome proliferator-activated receptor gamma; TNF-α, tumor necrosis factor alpha.
3.6. Sample Size
In this study, we used a randomized clinical trial sample size calculation formula using power analysis software, where type one (α) and type two errors (β) were 0.05 and 0.20 (power = 80%), respectively. According to our previously published trial (16), we used 0.15 fold changes as the SD and 0.15 fold changes as the change in mean (d) of TNF-α as a primary outcome. Based on the formula, we needed 16 patients in each group. After allowing for 20% dropouts in each group, the final sample size was 20 persons in each group.
3.7. Statistical Analysis
The normality of data distribution was assessed using Kolmogorov-Smirnov test. Independent sample t-test was applied to determine the differences in anthropometric measures and dietary intakes between the two intervention groups. Ratios differences were evaluated using Fisher’s exact test. To determine the effects of vitamin D administration on gene expression influenced by inflammation and insulin, we used independent sample t-test. P values < 0.05 were considered statistically significant. All statistical analyses performed using the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, Illinois, USA).
4. Results
Twenty patients in both groups completed the trial out of 45 patients who were recruited in our study (5 patients were excluded from the study because of not meeting the inclusion criteria). On average, higher than 90% of capsules were taken in both groups. No serious adverse effects were reported following the taking of vitamin D in patients.
Mean age, height, weight, body mass index (BMI) and COWS at baseline and end-of-trial, age of first use, route of use of illegal opioid, type of drug, methadone dose, duration of MMT, psychiatric comorbidity and use of other drugs were not significantly different between the two groups (Table 2). After the 12-week intervention, serum 25(OH) vitamin D levels significantly increased in the intervention group compared with the placebo group (+8.5 ± 5.1 vs. -0.8 ± 3.0, P < 0.001).
| Placebo Group | Vitamin D Group | Pb | |
|---|---|---|---|
| Age, y | 43.4 ± 9.8 | 40.7 ± 6.4 | 0.30 |
| Height, cm | 172.7 ± 7.7 | 170.5 ± 8.7 | 0.40 |
| Weight at study baseline, kg | 77.1 ± 12.2 | 71.9 ± 11.4 | 0.17 |
| Weight at end-of-trial, kg | 77.0 ± 13.0 | 71.8 ± 11.8 | 0.19 |
| Weight change, kg | -0.1 ± 1.9 | -0.1 ± 1.3 | 0.98 |
| BMI at baseline, kg/m2 | 25.9 ± 3.8 | 24.8 ± 3.8 | 0.39 |
| BMI at end-of-trial, kg/m2 | 25.8 ± 4.2 | 24.7 ± 3.9 | 0.41 |
| BMI change, kg/m2 | -0.1 ± 0.6 | -0.1 ± 0.4 | 0.95 |
| Vitamin D at baseline, ng/mL | 13.8 ± 4.5 | 14.7 ± 3.7 | 0.49 |
| Vitamin D at end-of-trial, ng/mL | 13.0 ± 6.4 | 23.2 ± 7.0 | < 0.001 |
| Vitamin D change, ng/mL | -0.8 ± 3.0 | 8.5 ± 5.1 | < 0.001 |
| Withdrawal symptoms at baseline | 27.6 ± 7.5 | 24.1 ± 13.1 | 0.30 |
| Withdrawal symptoms at end-of-trial | 25.9 ± 7.9 | 21.4 ± 8.1 | 0.08 |
| Withdrawal symptoms change | -1.6 ± 4.3 | -2.7 ± 9.7 | 0.67 |
| Age of first use, y | 22.8 ± 8.9 | 22.4 ± 8.8 | 0.88 |
| Education, % | 0.63c | ||
| Illiterate | 10 (50) | 8 (40) | |
| Elementary | 3 (15) | 4 (20) | |
| Intermediate | 5 (25) | 7 (35) | |
| Diploma | 2 (10) | 1 (5) | |
| Marital status, % | 0.75c | ||
| Single | 12 (60) | 10 (50) | |
| Married | 4 (20) | 3 (15) | |
| Widow/divorced | 4 (20) | 7 (35) | |
| Job, % | 0.81c | ||
| Unemployed | 13 (65) | 9 (45) | |
| Employed | 0 (0) | 1 (5) | |
| Others | 7 (35) | 10 (50) | |
| Route of use of illegal opioid, % | 0.91c | ||
| Smoking | 6 (30) | 7 (35) | |
| Smoking and injecting | 5 (25) | 4 (20) | |
| Mix | 9 (45) | 9 (45) | |
| Type of drug, % | 0.88c | ||
| Poly-drug users | 13 (65) | 14 (70) | |
| Opium and opium residues | 4 (20) | 4 (20) | |
| Opium and heroin | 3 (15) | 2 (10) | |
| Methadone dose, mL/d | 18.5 ± 6.1 | 18.7 ± 6.8 | 0.90 |
| Duration of MMT, y | 6.6 ± 2.8 | 5.7 ± 2.0 | 0.24 |
| Psychiatric comorbidity, % | 0.88c | ||
| None | 14 (70) | 12 (60) | |
| Mood disorder | 2 (10) | 2 (10) | |
| Anxiety disorder | 2 (10) | 2 (10) | |
| Mood and anxiety disorders | 1 (5) | 3 (15) | |
| Others disorders | 1 (5) | 1 (5) | |
| Use of other drugs | 1.00c | ||
| None | 13 (65) | 13 (65) | |
| Benzodiazepine | 7 (35) | 7 (35) |
aValues are expressed as mean ± SD or No. (%).
bObtained from independent t-test.
cObtained from Fisher’s exact test.
Considering the 3-day dietary records obtained during the intervention, there was no statistically significant difference in terms of dietary macro- and micro-nutrient intakes between vitamin D and placebo groups (data not shown).
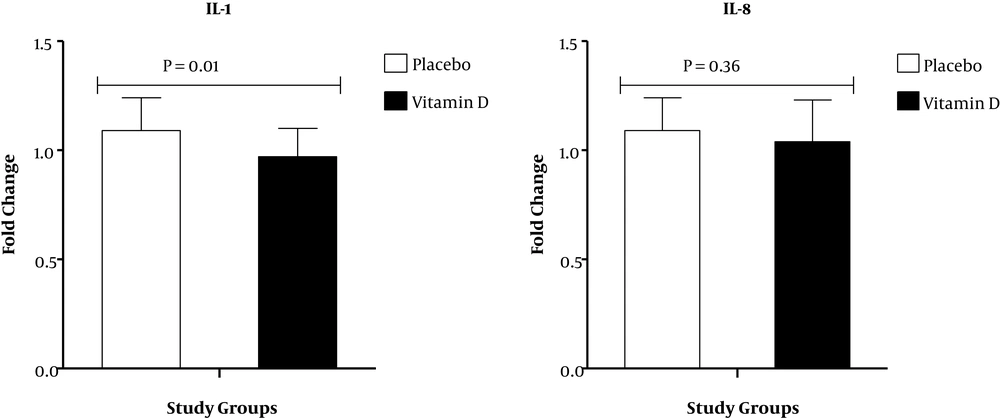
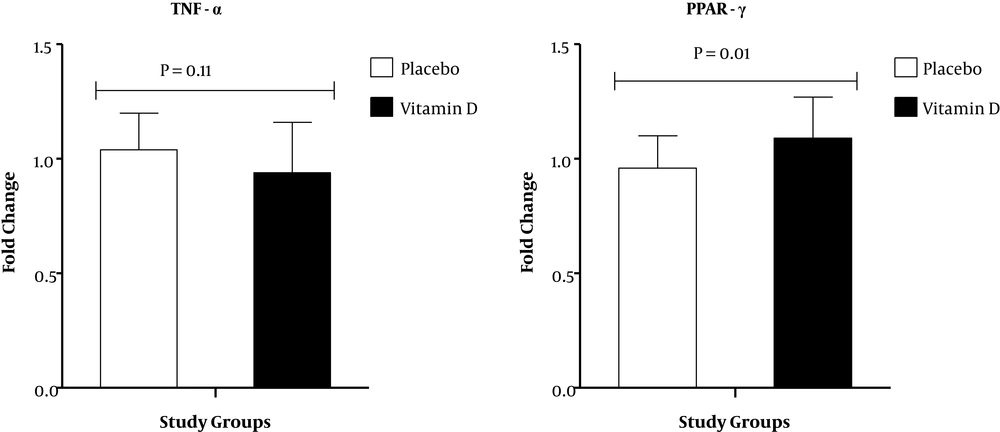
Results of RT-PCR demonstrated that compared with the placebo, vitamin D supplementation down-regulated gene expression of IL-1 (P = 0.01) in PBMCs of patients under MMT (Figure 1). There was no significant effect of vitamin D supplementation on gene expression of IL-8 and TNF-α in PBMCs of patients under MMT (Figures 1 and 2). Also, compared with the placebo, vitamin D supplementation up-regulated gene expression of PPAR-γ (P = 0.01) in PBMCs of patients under MMT (Figure 2). We adjusted the analysis for age and BMI at baseline. When we adjusted the analysis for age and baseline BMI, our findings did not alter (Table 3). In addition, when we controlled the analysis for age, BMI at baseline, duration of MMT and psychiatric comorbidity, our findings did not alter.
| Placebo Group | Vitamin D Group | Pb | |
|---|---|---|---|
| IL-1 (fold change) | |||
| Model 1c | -50.2 ± 23.4 | 78.3 ± 23.4 | < 0.001 |
| Model 2d | -40.5 ± 23.3 | 68.7 ± 23.2 | 0.002 |
| IL-8 (fold change) | |||
| Model 1c | -37.3 ± 16.6 | -6.9 ± 16.6 | 0.20 |
| Model 2d | -43.3 ± 19.3 | -0.9 ± 19.3 | 0.13 |
| TNF-α (fold change) | |||
| Model 1c | 0.5 ± 0.2 | -0.1 ± 0.2 | 0.05 |
| Model 2d | 0.5 ± 0.2 | -0.1 ± 0.2 | 0.08 |
| PPAR-γ (fold change) | |||
| Model 1c | 0.5 ± 0.5 | -2.1 ± 0.5 | < 0.001 |
| Model 2d | 0.5 ± 0.5 | -2.1 ± 0.5 | < 0.001 |
Abbreviations: IL-1, interleukin-1; IL-8, interleukin-8; PPAR-γ, peroxisome proliferator-activated receptor gamma; TNF-α tumor necrosis factor alpha.
aValues are expressed as mean ± SD.
bObtained from ANCOVA.
cModel 1: Adjusted based on age, BMI at baseline and baseline values of gene expression of variables.
dModel 2: Adjusted based on age, BMI at baseline and baseline values of gene expression of variables, type of drug, duration of MMT and psychiatric comorbidity.
5. Discussion
We assessed the favorable effects of vitamin D intake for 3 months on withdrawal symptoms and gene expression associated with inflammatory cytokines and insulin subjects under MMT. This study documented that vitamin D administration for 3 months among MMT subjects, compared with the placebo, ameliorated IL-1 and PPAR-γ expression, but did not impact IL-8 and TNF-α expression and COWS. This is the first investigation reporting the effects of vitamin D administration on withdrawal symptoms and gene expression related to metabolic outcomes among patients under MMT. It must be kept in mind that a number of studies have shown that patients treated with methadone experience withdrawal symptoms that the onset of withdrawal syndrome lasts from 7 to 10 days and these symptoms continue up to few weeks (22). The most common reason for successful termination of MMT treatment was the belief that they needed the higher methadone dose and has experiences the withdrawal symptoms. Although we believe that these symptoms are not common in long-term periods; further studies are needed to confirm our findings.
Of note, MMT leads to some disorders, including increased inflammatory cytokines (4). A number of studies have documented that hypovitaminosis D and BMD are difficulties in patients under MMT (9, 23). In a study of subjects with chronic pain, 93% of them had colecalciferol deficiency (24). Authors documented that vitamin D intake for 3 months in MMT subjects could not improve withdrawal symptoms. The effects of vitamin D intake on withdrawal symptoms among subjects under MMT are limited. Several studies have shown that hypovitaminosis D may be important in addiction and may have an effect in destabilizing serotoninergic, dopaminergic, and glutamatergic pathways, which subsequently contribute to the degenerating mental state (25). In addition, vitamin D indirectly interferes with neuron membrane integrity, as well as its function, which may improve neuron malfunction (26).
We showed that consuming vitamin D for 3 months downregulated IL-1 expression, but could not affect IL-8 and TNF-α expression. Previously, the effect of vitamin D administration on inflammatory factors was evaluated in patients without MMT. In agreement with our study, vitamin D significantly reduced IL-1β expression in macrovascular endothelial cells compared with non-treated cells (27). The same findings were reported by other studies (16, 28). However, a bolus dose of vitamin D (250,000 IU) in subjects with cystic fibrosis was related to a reduction in two inflammatory markers, IL-6 and TNF-α, but did not affect IL-1 and IL-8 (29). Pro-inflammatory markers directly oppose opioid actions (4). In addition, another study showed that pro-inflammatory markers, including IL-1β and TNF-α were related to enhancing pain intensity (30). Increased inflammatory variables may play an active role in the progress of many metabolic disturbances, such as type 2 diabetes mellitus or atherosclerosis progression leading to cardiovascular diseases (31). Colecalciferol may decrease inflammatory variables via regulation of nuclear factor κB (NF-κB) and mitogen-activated protein (MAP) kinase (32).
We found that vitamin D administration to patients under MMT for 3 months upregulated PPAR-γ expression. Previously, impaired insulin metabolism was demonstrated in MMT compared with control group (33). Moreover, circulating levels of leptin, adiponectin and resistin significantly change in individuals with chronic heroin addiction (34), which in turn would result in decreasing insulin sensitivity. In accordance with these results, Hoseini et al. (13) observed that combined high-dose vitamin D and aerobic exercise for 2 months significantly increased PPAR-γ expression in the liver. Also, it was documented that hypovitaminosis D resulted in the dysregulation of insulin sensitivity by reducing adipose PPAR-γ expression and deteriorating β-cell function (14). In addition, gene expression of PPAR-γ was upregulated by vitamin D in tissue of pregnant rats, but its expression was not significantly increased in the liver (35). However, this finding differs from prior studies on pre-adipocytes, which demonstrated that vitamin D considerably downregulated the C/EBP-α and PPAR-γ expression (36). Overall, our results suggest that vitamin D modulate insulin and lipid metabolism by blocking the expression of lipogenic enzymes in adipose tissue and the liver. The important role of PPAR-γ in regulating motivated behaviors and emotional control is documented with neuroanatomical data, showing relatively high concentrations of receptor expression in neurons of the caudate-putamen, septum, and hypothalamus (37). A prior study demonstrated that the activation of the PPAR-γ led to inhibition of stress response in rodents (38). Currently, effective drugs for addiction treatment are methadone and buprenorphine. Therefore, existing evidence suggests that activating PPAR-γ may represent a promising effective treatment approach for drug abuse (39).
5.1. Limitations
The present study had some limitations. Firstly, duration of this study was short. Long-term duration may lead to better effects in withdrawal symptoms. Secondly, we did not evaluate the effects of colecalciferol on cognitive function. Thirdly, we could not evaluate the acute and chronic pain in patients under MMT. In addition, vitamin D and placebo were produced by two different companies. This should be considered in the interpretation of our findings.
5.2. Conclusions
Totally, taking vitamin D for 3 months in patients under MMT significantly ameliorated the IL-1 and PPAR-γ expression, but did not affect IL-8 and TNF-α expression and COWS. Vitamin D can be recommended as an adjunct to MMT in opioid withdrawal protocol which may elevate the quality of life and may diminish methadone adverse effects.