1. Background
It is now clear that chronic stress can lead to anxiety in human and animal models (1). It is proposed that glucocorticoid hormones released during chronic stress can increase glutamate system activity in the basolateral amygdala (BLA), and ultimately, induce neuronal sensitization in the region (2-5). The resulting synaptic plasticity which can increase BLA glutamatergic and GABAergic outputs to the ventral hippocampus and prefrontal cortex, in fact, leads to anxiety in animals (6, 7).
Previous researches have shown that glutamatergic inputs from different brain areas are projected to BLA pyramidal neurons (8). Glutamate interacts with its inotropic glutamate receptors, e.g. NMDA, and triggers synaptic plasticity processes such as long term potentiation-LTP and/or stimulates BDNF expression in these neurons (9, 10). According to these findings, it is hypothesized that administration of NMDA receptor antagonists in BLA, as well as in areas associated with BLA such as dorsal and ventral hippocampus and prefrontal cortex, may have anxiolytic effect (9, 11, 12). Moreover, both peripheral and central injections of the nonspecific NMDA receptor antagonist, MK-801, in rats reduce anxiety as evaluated by elevated plus maze (EPM) (9, 13, 14). Further, another NMDA receptor antagonist, AP-7, either peripherally (9, 15) or centrally (16), reduces anxiety caused by exposure to the predator or water deprivation in rats. Other studies have shown that administration of memantine as an NMDA receptor antagonist reduces Parkinson’s disease-induced anxiety (17). Consistent with these studies, it has been shown that memantine may be effective in treating patients with treatment-resistant anxiety (18). In one study, it was found that administration of NMDA as an agonist of NMDA glutamate receptors into the periaqueductal gray matter (PAG) induced anxiety-like behaviors in rats (19). Studies have also indicated that administration of glucocorticoids such as dexamethasone and corticosterone, peripherally, intra-hippocampus and intra-BLA, may lead to anxiety-like behaviors or facilitate these behaviors (20-23).
On the other hand, studies have indicated that hippocampal BDNF level is reduced in animal models of anxiety (24-26). It is interesting that BDNF concentration is also reduced in the plasma and cerebrospinal fluid (CSF) of humans suffered from anxiety (27-30). Interestingly, it has been shown that administration of NMDA receptor antagonists can increase BDNF in the hippocampus of stressed rats (31-33). It has also been shown that the plasma and CSF concentration of BDNF is extremely low in patients suffered from major depression, but increases after treatment with fluoxetine or ketamine as another NMDA receptor antagonist (34-36).
2. Objectives
The present study investigated the effect of inhibition of NMDA glutamate receptors on maintenance of anxiety-like behavior and hippocampal BDNF level in rats subjected to electro foot shock stress for seven consecutive days, followed by a six-day interval period. For this purpose, intra-BLA injections of memantine were performed before each stress session either intraperitoneally or intra-BLA. The open field test was chosen because of its validity for evaluation of normal and pathological anxiety (37). It needs to be explained that since BLA is one of the areas which directly receives sensory noxious inputs (38), electric foot shock stress was used for stress induction.
3. Methods
3.1. Drugs
Memantine hydrochloride (Sigma-USA), ketamine hydrochloride (Alphason-Netherlands), and diazepam hydrochloride (Sigma-USA) were used in the study. All the drugs were dissolved in sterile saline and used at a volume of 1 mL/kg (peripheral) or 0.25 μL/side (centrally).
3.2. Animals
Male Wistar rats (W: 180 - 210 g) purchased from the Pasteur Institute (Tehran, Iran) were used in the study (n = 6 - 8/group). The animals were kept in four cages with free access to tap water and rat chow (Pars Animal Food Co., Tehran, Iran), at 12-h light/dark intervals and 21 - 24ºC. Each animal was used once. The procedure time line of the experiments is shown in Figure 1. All the experiments took place between 9:00 and 16:00. The animals were handled and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (1996, published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA) and approved by the Committee on the Use and Care of Animals of Baqiyatallah University of Medical Sciences, with the approval code #87/381.
3.3. Surgical Procedures
The animals were anesthetized using diazepam (4 mg/kg; ip) and ketamine (60 mg/kg; ip) anesthesia. Two cannulas (stainless steel gauge 23) were placed in BLA using stereotaxic coordination (L = ±5, AP = -2.7, and D = 8.5 mm) (39). The cannulas were designed to be placed 500 µm above BLA. To avoid the closure of the cannulas, a 0.3 mm diameter stainless steel filament was placed inside each cannula. The animals were left for seven days for recovery before the experiments began. For bilateral intra-BLA memantine injection, a 30-gauge dental needle was used, which was connected to a 1 µL Hamilton syringe via a poly ethylene tubing system. The drug was injected into BLA in a volume of 0.25 µL/side. After completion of the experiments, the animals were deeply anesthetized by ketamine (50 mg/kg; ip) and their brains were removed through a trans-cardiac surgical procedure. The brains were dissected, and the place of injection was verified by a professional experimenter. Only those animals which had the correct place of injection were considered for statistical analysis.
3.4. Stress Induction Procedure
Stress was induced by a communication box (CB) device (Borj-e-Sanat Co, Tehran, Iran). The apparatus consists of nine separate compartments (16 × 16 × 40 cm; length × width × height) made by Plexiglas. The floor of the apparatus was made by stainless steel grids (4 mm diameter and 13 mm spaced). The grids were connected to a computer programmed electrical current generator, which controlled both the intensity and duration of the electrical shock by user (40). The generator provided intermittent electric foot shocks (1.2 mA, 10 Hz and 10 sec duration). Each animal received 100 electrical stimuli in 10 sec. The experiments were carried out between 10:00 - 13:00 for seven consecutive days. For stress induction, the animals were transferred to the test room one hour before the experiment for the purpose of environmental adaptation. Then, each animal was placed in one compartment separately and remained in the compartment for 30 min without electrical shock. Afterward, they experienced the shock for 10 sec and remained in the apparatus for an additional 30 min. The control groups also received the drug or saline and were placed in the compartments for 60 min without inducing shock. Stress induction continued for seven consecutive days.
3.5. Open Field Testing Method
The test began six days after stress termination when the animals were in drug free state. Each open field experimental session lasted for 5 min. The open field device was made from black Plexiglas with dimensions of 36 × 36 × 36 cm (length, width and height, respectively). A video camera connected to the PC recorded and analyzed the animals’ activities using the Auto Vision software (Borj-e-Sanat Co., Tehran, Iran). The software can virtually divide the floor of the apparatus to 16 equal sections (4 central and 12 peripheral zones). Animal locomotion (in cm), number of cases entering the central zone, and time spend in the central zone were recorded. These parameters were considered as the indicators of anxiety in the animals (12). Moreover, thigmotaxis, as an index for definition the influence of tactile sensory inputs on animal preference to spend their time in the periphery with their anxiety was calculated as follows: ratio of (the number of entries into the central part/rat locomotor activity) × 1000. The high value of the score led to low thigmotaxis as well as to the great pronounced anxiolytic-like effect (41-43).
3.6. Study Design
3.6.1. The Effect of Intraperitoneal Memantine Injection on Anxiety-Like Behavior in Stressed Rats
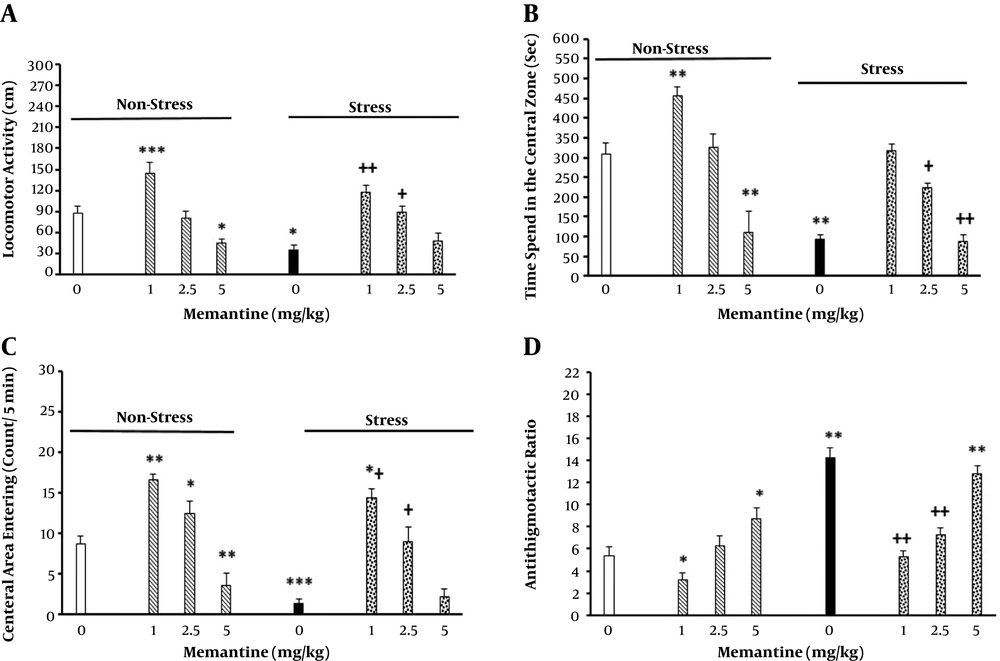
Eight animal groups were used (n = 6 - 8/group), of which three groups received different doses of memantine (1, 2.5, and 5 mg/kg; ip) 30 min before being placed in CB for 60 min while the apparatus was off (44). In addition, three groups received the same memantine doses, were placed in CB and received electric foot shock as described earlier. The procedure was repeated for seven consecutive days. The control groups received saline (1 mL/kg). Maintenance of stress-induced anxiety was tested six days later, as described earlier in this section. Results are shown in Figure 2.
The effect of peripheral memantine injection on locomotor activity (A), elapsed time (B), number of entries into the central zone (C), and the thigmotaxis ratio (D) in the non-stressed and stressed rats. The animals first received different doses of memantine (1, 2.5, and 5 mg/kg; ip) or saline 30 min before stress induction for seven consecutive days. Six days later, their behavior was recorded in the open field apparatus for five min (n = 6 - 8/group). *P < 0.05, **P < 0.01, ***P < 0.001 different from control groups. +P < 0.05, ++P < 0.01 different from the stress group.
3.6.2. The Effect of Bilateral Intra-BLA Memantine Injection on Anxiety-Like Behavior in Stressed Rats
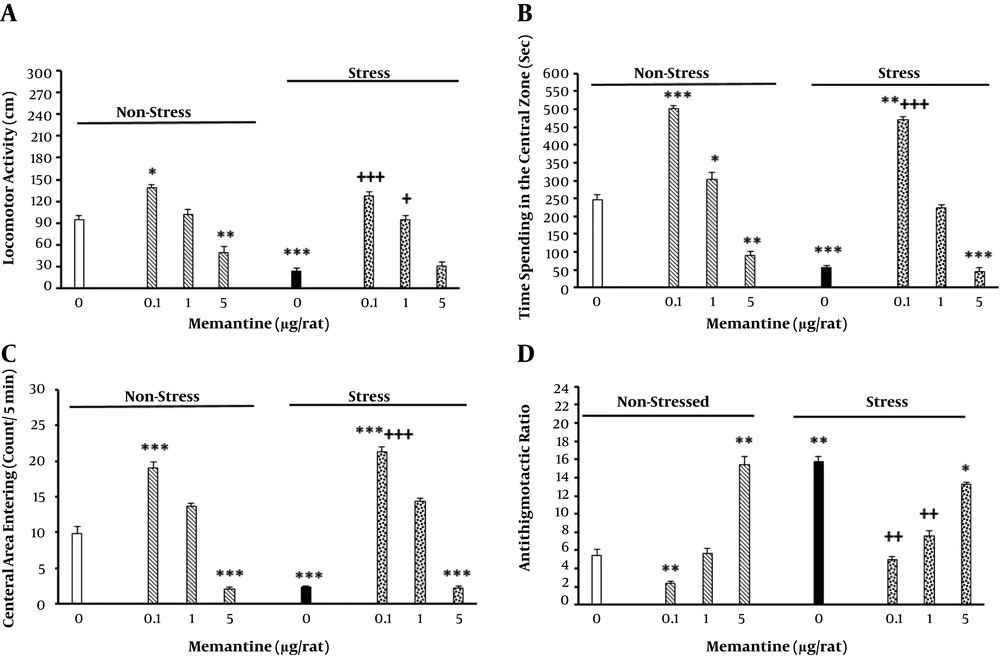
Eight groups were selected (n = 6 - 8/group) for this propose. Three groups received different doses of memantine (0.1, 1, 5 and 5 μg/rat; intra-BLA) bilaterally, 5 min before being placed in the apparatus without shock induction for seven consecutive days (44). Moreover, three groups received the same doses of the drug, were placed in CB and received electric foot shock. The control groups received saline (0.25 μL/side). The results are shown in Figure 3.
The effect of intra-BLA memantine injection on locomotor activity (A), elapsed time (B), number of entries into the central zone (C), and the thigmotaxis ratio (D) in the non-stressed and stressed rats. The animals first received different doses of memantine (0.1, 1, and 5 μg/rat) or saline (0.25 μL/side) 5 min before stress induction for seven consecutive days. Six days later, their behavior was recorded in the open field apparatus for 5 min (n = 6 - 8/group). *P < 0.05, **P < 0.01, ***P < 0.001 different from control groups. +P < 0.05, ++P < 0.01, +++P < 0.001 different from the stress group.
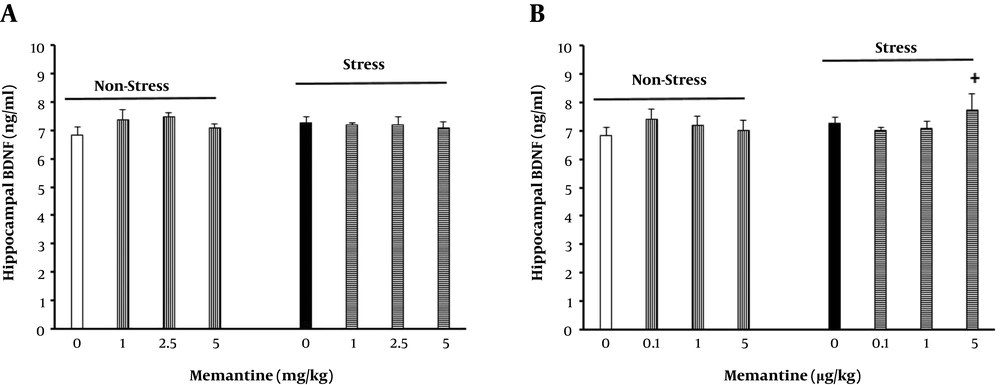
3.6.3. The Effect of Intraperitoneal and Intra-BLA Memantine Injection on Hippocampal BDNF Level in Stressed and Non-Stressed Rats
Sixteen groups were selected (n = 6/group) for this propose. From the selected groups, six received different doses of memantine (1, 2.5 and 5 mg/kg; ip or 0.1, 1, 5 and 5 μg /rat; intra-BLA) 30 and 5 min, respectively, before being placed in the apparatus for seven consecutive days. Furthermore, six groups received the same doses of the drug, were placed in CB and received electric foot shock. The control groups received saline (1 mL/kg and 0.25 μL/side, respectively). Six days later, the animals were deeply anesthetized and their brains were taken out after cold (4ºC) saline injection via a trans-cardiac operation. The brains were then sectioned in a brain matrix apparatus, and the hippocampus was removed and frozen at -80ºC for BDNF level evaluation. Results are shown in Figure 4.
The effect of peripheral and intra-BLA memantine injection on hippocampal BDNF level in the rats. The animals first received different doses of memantine (1, 2.5 and 5 mg/kg or 0.1, 1, and 5 μg/rat respectively) or saline (0.25 μL/side or 1ml/kg) 5 or 30 min, respectively, before stress induction for seven consecutive days (n = 6/group). +P < 0.05 different from the stress group.
3.7. Statistical Analysis
The data were expressed as mean ± standard error of mean (SEM) of the variables. Two-way ANOVA, followed by Newman-Keuls multiple comparisons as the post hoc test, was used to analyze the data using stress and the drug as the variables. A P value of less than 0.05 was considered statistically significant.
4. Results
4.1. The Effect of Intraperitoneal Memantine Injection on Anxiety-Like Behavior in Stressed Animals
The results indicated that stress reduced the total locomotion of the animals, time elapsed in the central zone, number of entries into the central zone and thigmotaxis. In addition, increasing memantine in the non-stressed animals decreased locomotor activity, time elapsed in the central zone, number of entries into the central zone and thigmotaxis in a dose-dependent manner (Figure 2). However, intraperitoneal administration of memantine in the stressed animals neutralized the effects of stress on the above mentioned parameters. The resulted statistical analysis for distance [2-way ANOVA, intra-group comparison: memantine effect: F (7, 40) = 3.22, P < 0.001, stress effect: F (1, 40 ) = 4.1, P < 0.001, memantine × stress interaction: F (7, 40) = 4.32, P < 0.001] (Figure 2A), elapsed time [2-way ANOVA, intra-group comparison: memantine effect: F (7, 40) = 2.75, P < 0.001, stress effect: F (1, 40) = 3.2, P < 0.001, memantine × stress interaction: F (7, 40) = 2.56, P < 0.001] (Figure 2B), frequency of entries into the central part of the device [2-way ANOVA, within-group comparison: memantine effect: F (7, 40) = 4.12, P < 0.001, stress effect: F (1, 40) = 3.2, P < 0.001, memantine × stress interaction: F (7, 40) = 5.17, P < 0.001] (Figure 2C), and thigmotaxis [2-way ANOVA, intra-group comparison: memantine effect: F (7, 40) = 2.38, P < 0.001, stress effect: F (1, 40) = 1.987, P < 0.01, memantine × stress interaction: F (7, 40) = 2.1, P < 0.001] (Figure 2D).
4.2. The Effect of Bilateral Intra-BLA Memantine Injection on Anxiety-Like Behavior in Stressed Rats
The results showed that the increased injection of memantine into BLA in the non-stressed animals decreased locomotor activity, time elapsed in the central zone, number of entries into the central zone, and thigmotaxis in a dose-dependent manner (Figure 3). However, intra-BLA memantine administration in the stressed animals prevented the effects of stress on the above mentioned parameters. The statistical analysis was obtained for locomotor activity [2-way ANOVA, intra-group comparison: memantine effect: F (7, 40) = 2.61, P < 0.001, stress effect: F (1, 40) = 3.9, P < 0.001, memantine × stress interaction: F (7, 40) = 3.76, P < 0.001] (Figure 3A), time elapsed in the central zone [2-way ANOVA, intra-group comparison: memantine effect: F (7, 40) = 3.1, P < 0.001, stress effect: F (1, 40) = 5.21, P < 0.001, memantine × stress interaction: F (7, 40) = 4.52, P < 0.001] (Figure 3B), the frequency of entries into the central part [2-way ANOVA, within-group comparison: memantine effect: F (7, 40) = 4.26, P < 0.001, stress effect: F (1, 40) = 3.78, P < 0.001, memantine × stress interaction: F (7, 40) = 5.23, P < 0.001] (Figure 3C), and thigmotaxis [2-way ANOVA, intra-group comparison: memantine effect: F (7, 40) = 2.01, P < 0.01, stress effect: F (1, 40) = 2.61, P < 0.01, memantine × stress interaction: F (7, 40) = 2.9, P < 0.01] (Figure 3D).
4.3. The Effect of Intraperitoneal and Intra-BLA Memantine Injection on Hippocampal BDNF Levels in Stressed and Non-Stressed Animals
The results also showed that the IP and intra-BLA injections of memantine in the non-stressed and stressed animals had no statistically significant effect on hippocampal BDNF level (Figure 4).
5. Discussion
The present study was conducted to investigate the role of NMDA glutamate receptors located in BLA on maintenance of chronic stress-induced anxiety. To this end, the open field test was performed. The reason for not using EPM for our evaluation is that EPM which is generally used in studies related to anxiety is thought to be influenced by experimental conditions such as pre-exposure to stressful events (37, 45). In this regard, some investigators also believe that the open field method may better show anxiolytic effects of drugs (12, 43), which is better fitted with the aims of this study, i.e. the anxiogenic effects of stress and/or memantine.
In the present study, it was observed that stress could lead to anxiety-like behavior. This was indicated by the increased time spent by the animals in the peripheral zone of the device, the decreased number of entries into the central zone, and the increased distance traveled at the peripheral zone of the device. Interestingly, both peripheral and intra-BLA memantine administrations also increased anxiety in the non-stressed animals. However, the drug reduced maintenance of stress-induced anxiety in the stressed animals when administered both peripherally and centrally. This indicated that the effects of stress and the drug remained on anxiety maintenance at least six days after termination of stress and the drug administration.
Several studies have shown that various stresses can increase release of glucocorticoid hormones (e.g., corticosterone and cortisol) as a result of HPA axis activity (46). These hormones affect their receptors located in the cell cytoplasm of the neurons within CNS for induction of their effects (47). Studies have indicated that receptors for glucocorticoids are abundant in the cytoplasm of BLA neurons, and their activity may result in increasing dendritic arborization of these neurons (4, 5, 48). This activity also increases synaptic spines on dendrites of these neurons (49). Together, these morphological changes are in line with increased function of these neurons (50). In this regard, previous studies have shown that morphological changes induced by stress can be traced in BLA neurons 21 days after stress termination (47). Hypertrophic morphological changes as well as neuronal activity of BLA neurons is postulated to be related to increased anxiety-like behavior observed in chronically stressed animals (51). On the other hand, NMDA glutamate receptors located on the plasma membrane of neurons in the BLA region have been shown to play an important role in neuroplasticity of glutamatergic synapses and, consequently, in synaptic activity enhancement in these regions (52). The current research showed that six days after stress induction, the rats still showed anxiety, which is in agreement with the above mentioned data.
In the next part of this investigation, the findings showed that both peripheral and intra-BLA memantine administrations led to anxiety-like behavior in the non-stressed rats, which was more pronounced in the intra-BLA treated animals. In other words, chronic inhibition of NMDA receptors, especially in BLA, may induce anxiety in animals. According to these data, one can conclude that NMDA receptors within BLA may have a pivotal role in this regard. However, the exact mechanism, by which chronic memantine administration induces anxiety, is not clear. Other studies have shown that acute glutamate NMDA receptor agonist injections in the hippocampus can induce anxiety in rats (52). However, so far, little research has focused on the effects of NMDA receptors located in BLA in this regard. The findings have also a clinical significance, considering that the drug is currently used in humans for treatment of mild to moderate Alzheimer’s disease (17). The findings of the present study may show at least a degree of hazard for chronic memantine use. It is recommend to further investigate anxiety induced by chronic memantine administration by using other methods such as EPM and the dark-light test. Moreover, it might be helpful to study cellular and molecular changes induced by chronic memantine, especially in BLA.
Interestingly, pre-administration of memantine before each stress session reduced maintenance of stress-induced anxiety. At first, it appears to be a controversial result since the data showed that chronic memantine administration induced anxiety. However, the discrepancy may be resolved by considering that the drug induces anxiety by itself when administered in normal animals. However, it can inhibit stress by inhibition of glutamate receptors in the central nervous system when administered before a stress session. Accordingly, some studies have shown that administration of NMDA receptor antagonists can reduce anxiety in animal models. For example, peripheral (13, 14) and/or intra-hippocampal (9, 14) MK-801 (a glutamate NMDA receptor antagonist) injection reduces anxiety in non-stressed rats. In addition, it is shown that AP-7, as another NMDA antagonist, can reduce stress-induced anxiety when administered peripherally (9, 15) or centrally (16). In humans, it is also shown that memantine is useful for treatment of anxiety induced by Parkinson’s disease (17). Based on these data, it is not surprising that administration of NMDA, as an agonist of NMDA glutamate receptors, into PAG can induce anxiety in non-stressed rats (19). However, all relevant experiments use antagonists acutely or chronically, although data is insufficient on effects of these drugs on maintenance of stress-induced anxiety. In this regard, it is recommended to use other NMDA receptor antagonists such as MK-801 or AP-7 with different stress types including restraint stress and immobilization stress for further evaluation of the role of these receptors in maintaining chronic stress-induced anxiety.
In the last part of the experiments, the results showed that stress had no effect on hippocampal BDNF level. Moreover, both peripheral and intra-BLA memantine administrations had no effect on BDNF level in the hippocampus. In previous studies, it was revealed that hippocampal BDNF level decline was associated with anxiety in both human and animal models (29). Other studies indicated that BDNF level was reduced after chronic restraint stress in rats and mice (53, 54). The lack of change in BDNF level in the present study may be due to the time elapsed between termination of the stress sessions and hippocampal BDNF level assessments. In agreement with this hypothesis, studies indicated that stress-induced BDNF reduction in the hippocampus was reversed less than two days after stress termination (55-57). Thus, it is not surprising that we could not track any changes in hippocampal BDNF level six days after stress termination. Of course, it must be noted that the stress session in these studies was acute stress. Accordingly, it is concluded that chronic stress may have large effects on hippocampal BDNF level. Moreover, it is concluded that the time between stress termination and hippocampal BDNF assessments should be shorter than six days. It is suggested to investigate the effects of chronic stress on BDNF level in other brain regions including BLA.
It must be noted that only the NMDA receptor antagonist was used in the present study. Therefore, it is recommended to use the NMDA receptor agonist for further clarification of the role of these receptors in stress mediation.
5.1. Conclusions
Chronic electro foot shock stress or chronic memantine administration may induce anxiety in rats, remaining for at least six days after stress or drug administration termination, and BLA might be the site of stress or memantine action. In addition, memantine can inhibit maintenance of stress-induced anxiety. These results confirm the pivotal role of the NMDA receptor located in BLA in maintenance of anxiety induced by stress and memantine.