1. Background
Today, drug addiction is one of the most important social, psychological, and health problems around the world. Amphetamine is the second most commonly used drug, with nearly 35 million consumers (range, 13 to 58 million consumers) worldwide. The use of amphetamines, especially methamphetamine (meth), has increased in many areas of the world, including North America, Oceania, and most parts of Asia (1).
The long-term use of meth, as a highly addictive substance, can damage the dopaminergic, serotonergic, and metabolic systems of the brain, leading to cognitive impairments, such as memory, learning, attention, and concentration deficits (2, 3). Moreover, the impairment of executive functioning, planning, and decision-making, has been reported, besides mood disorders and anxiety, due to its neurotoxic effects on the brain (4).
Currently, there is no efficient or standard treatment for meth addiction (5). Therefore, in this type of addiction, similar to any other anxiety disorder, many scientific disciplines can be studied at anatomic, physiological, and molecular levels to find proper treatments (6). One of the most common methods is the use of animal models (7). The history of the use of laboratory animals provides convincing evidence to claim that animal studies have played a vital role in all medical advances. Therefore, it is necessary to develop and refine the animal models that can address important areas in biology, pathophysiology, clinical treatment, and drug screening for abuse liability.
Unlike clinical studies, the target population in preclinical models can be controlled for all unrealistic variables (8). Therefore, it is possible to design a study with fewer changes and interference effects. To design an appropriate animal model, it is necessary to develop the most similar model of diseases to human diseases by exerting the least amount of pain and stress on animals (9). Although it seems impossible to present a perfect model of diseases in animals, we should find the closest or the most appropriate model available.
To study the toxic effects of meth abuse, several animal models have been developed over the years. These models range from acute models with one exposure session to long-term models of chronic abuse (10). In general, there are different methods for the development of addiction models in rodents (Table 1). However, the best approach to developing animal models of addiction is the self-administration, in which the animal receives a drug by pressing an active lever in its cage. With the progression of addiction over time, drug dosage increases due to the animal’s tolerance according to its needs. Moreover, in this approach, one can analyze positive reinforcement, negative reinforcement, drug-seeking behaviors, and relapse rate (11).
| Different Animal Models of Addiction | Subgroups | Methods | Advantages | Disadvantages | Applications | Sources | |
|---|---|---|---|---|---|---|---|
| Acute model | Single-dose model | Drug injected at a single, very high dose, close to the toxic dose | Simple and time-consuming | Failure to develop an addiction model | A common examination method of oxidative stress, apoptosis, necrosis, and various enzymes, including caspases, in an organ or different organs after administering a toxic dose, followed by a behavioral test | Silva et al., 2014 | |
| One-day model | Injection of drug at a very high dose, close to the toxic dose (divided doses in one day) | Belcher et al., 2008 | |||||
| Chronic model | Fixed dosage | Prescription of a fixed amount of drug at regular intervals | Simplicity of modeling and improved similarity of the model to human addiction | Failure to observe addictive behaviors (e.g., craving for consumption, tolerance, and drug-seeking behaviors) | Development of models of addiction complications, such as memory and learning disabilities | North et al., 2013 | |
| Increased dosage | Prescription of drug according to a specific schedule by increasing the daily dose | Belcher et al., 2008 | |||||
| Self-administration | Intravenous injection (and intracranial injection) | Receiving drug by pressing an active lever in the cage | Development of an addictive model and observation of the main symptoms of addiction, such as drug-seeking behaviors | Difficulty of surgical and cannulation techniques | Cornett et al., 2013 | ||
| Inhalation | Voluntary entrance of the animal into the cage with a nebulizer and drug vapor | Development of an addictive model without the need for surgery and observation of the main symptoms of addiction, such as drug-seeking behaviors | Tim-consuming nature of modeling due to self-management training and increased amount of drug intake in modeling | The most similar model of animal addiction to human inhalant addiction | Juarez-Portilla et al., 2017 | ||
| Oral intake | Animal’s selection of two bottles of drug and water | Bitter taste of oral meth and time-consuming nature of modeling due to self-administration training | The most similar model of animal addiction to human oral addiction | Eastwood et al., 2014 | |||
Different types of self-administration include intravenous (IV) injection, oral intake, and inhalation. Intravenous self-administration, which is widely used today, is the typical method for developing an animal model of addiction similar to human addiction. The application of IV self-administration requires the high skill level of researchers in modeling addiction in laboratory animals. This technique is very difficult and requires the fixation of the cannula and catheter in the internal jugular vein behind the animal’s neck. To apply the intracranial injection method, the cannulation is performed on top of the animal’s head.
In oral self-administration, which is most commonly used for alcohol abuse, it is very difficult and time-consuming to model drugs with a bitter taste (e.g., meth). Therefore, a drawback of oral self-administration is that it is time-consuming. To overcome the shortcomings of current animal models of addiction and to develop a model of inhalation self-administration, a noninvasive model, highly similar to human models of addiction, was developed considering the rigorous techniques of available self-administration models (14).
More than 10% of meth users inject the drug intravenously, while some users utilize other routes of administration in addition to an IV injection, including nasal inhalation or snorting (91%) and smoking (28%). Since the inhalation of the meth vapor is the most common method (15), the development of an animal inhalation model seems necessary. Overall, animal models should be consistent with the characteristics of human diseases.
2. Objectives
Accordingly, we aimed to develop an inhalant addiction model with the greatest similarity to human meth addiction, using a simple, accurate, noninvasive, and low-cost method. We examined this model in terms of serum meth level and addictive behaviors, such as conditioned place preference (CPP) and locomotor activity. This novel model, with high validity, may increase the efficacy of translational research on meth addiction in humans.
3. Materials and Methods
3.1. Animals
Adult male rats, with an average weight of 200 - 210 g, were purchased from Royan Institute of Tehran, Iran, and kept under standard conditions in a 12:12 h light-dark cycle (8 a.m. to 8 p.m.) at 21 ± 3ºC with access to drinking water and food. The animals were transferred to the animal house for at least 10 days before the study; four rats were kept in the cage during this period. Through daily assessments, we made sure that none of the animals had diseases or evidence of diseases. The rats were, then, individually transferred to cages (27 × 20 × 14 cm) and randomly divided into two groups: control group (n = 8) and meth-addiction group (n = 7); one animal was lost due to unknown reasons after an initial meth inhalation session. The laboratory animals in the meth-addiction group were first placed in the self-administration training apparatus and learned to access the food by lever pressing; training continued for one to five days.
3.2. Drugs
Methamphetamine hydrochloride (Sigma) was dissolved in distilled water (DW) at a concentration of 1 mg/cc (5 mg/kg) in the first week of addiction and at 2 mg/cc (10 mg/kg) in the second week. By adjusting the apparatus, each time the lever was pressed, 50 μL of the solution was provided for inhalation. The maximum drug intake for each rat was 1 cc daily.
3.3. Self-Administration Apparatus
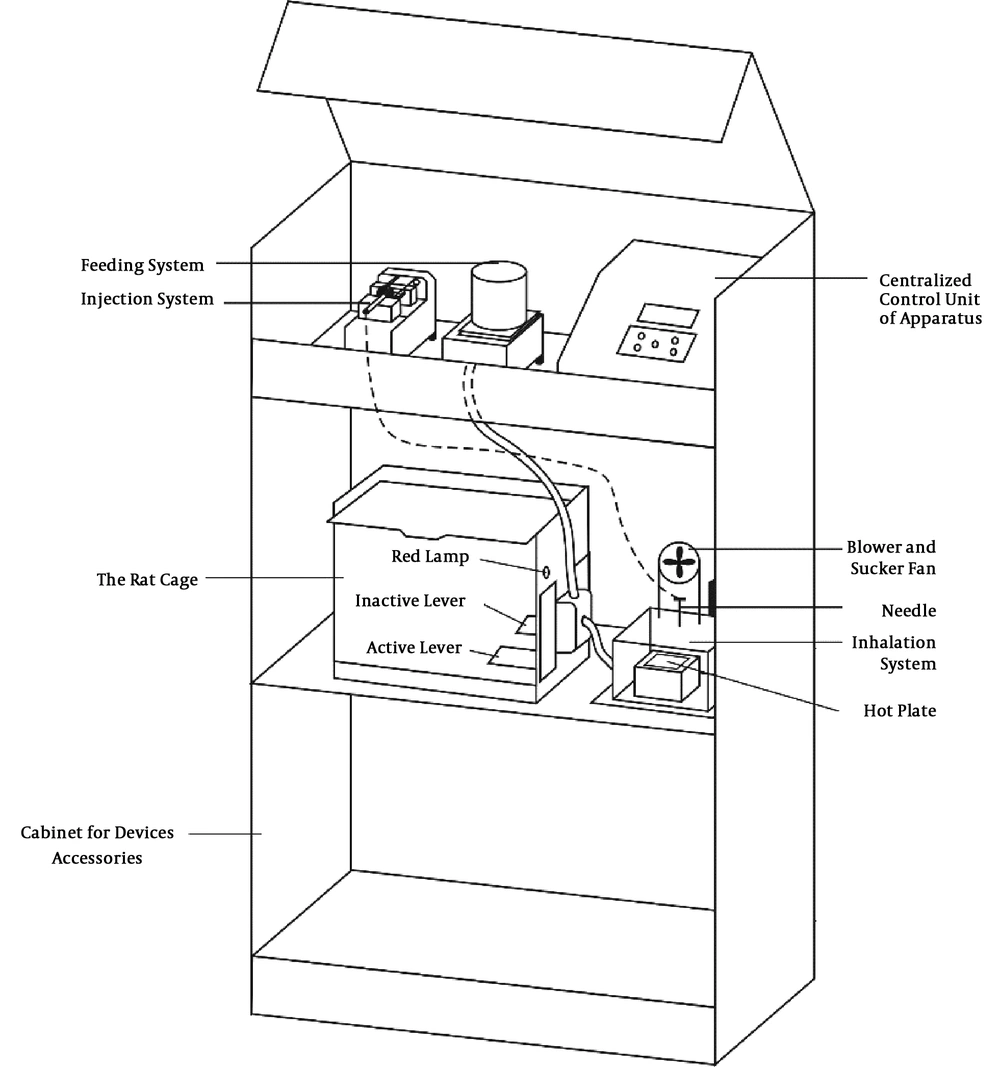
The apparatus for self-administration modeling in rodents constituted different parts (Figure 1). The cage, made of Plexiglass, was located in the middle of the apparatus. There were two levers in the cage, i.e., inactive and active. By pressing the passive lever, no drug was given to the rat, while by simultaneously pressing the active lever, a red light was illuminated and the rat received meth through inhalation at an adjusted amount. The cage had a ceiling cover without holes so that the drug vapor remained in the cage for some time to be inhaled, without being scattered in the laboratory.
In the inhalation self-administration part of the apparatus, a 3 - 5 cc syringe, containing a solution of meth with the specified concentration, was fixed in the automatic injection apparatus. As soon as the rat pressed the active lever, the drug was injected into a microinjection plastic tube at the concentration adjusted by the researcher. The injection volume of the drug (50 - 500 μL) and the interval between the injections (1 - 10 seconds) were adjustable.
To develop an inhalant addiction model, the drug solution (meth) was evaporated on a hot plate, adjacent to the rodent cage, and the vapor was blown by a fan into the rat cage. Two fans were used in this apparatus. The sucking fan started operating as soon as the inhalation system was activated; it sucked hot air from the hot plate compartment; therefore, the animals were not affected by the hot air from the plate. After drug injection, the blower fan was run for 15 s and then, the sucking fan was turned on again. To prepare the apparatus, about 10 min before placing the animal inside the cage, the inhalation method was applied in the setting section to reach the desired temperature during the test and evaporate the drug solution immediately.
The adjustment part of the apparatus was located at the top of the apparatus. The researcher could adjust the amount of inhalable or injectable drug (range, 50 - 500 μL), inter injection intervals (1 - 10 seconds), the number of times the lever was pressed, the use or non-use of feeding section, the number of times the active lever was pressed for food intake, and the total operating time of the apparatus for each animal. After the end of the experiment, the assessor provided a screening report for each animal to determine the number of times the active and passive levers were pressed for food intake and inhalation.
3.4. Self-Administration Training
First, the animals received self-administration training. For this purpose, they were deprived of food for 24 h and then placed in the apparatus for 4 h per day when they were hungry. The animals learned how to collect food after they searched the cage and pressed the levers; the duration varied from one to five days for laboratory animals. The red light was illuminated by pressing the active lever and receiving food. Using a few trials and errors, the animals were conditioned and learned to use the active lever. This lever was then used to deliver drug during the drug addiction period by establishing drug-seeking behaviors. After self-administration training, the rats were placed in the apparatus only to receive the drug. The experiments started with low doses of meth for inhalation (1 mg/1 cc). Evaluation of the amount of drug used by each animal in the apparatus indicated an increase in drug dosage through self-administration.
3.5. Meth Self-Administration Protocol
After the training period, all animals in the two groups were placed in the self-administration apparatus to initiate meth (meth-addiction group) or DW (control group) self-administration through inhalation (15 min/day). Pressing the active lever resulted in infusion, and the meth dosage reduced at 0.05 mg/kg in a volume of 50 μL (0.05 mL) within two seconds. Responses on the inactive lever were also recorded although they had no implications.
During the second week of self-administration via inhalation, the addicted rats were allowed to self-administer meth (0.1 mg/kg/50 μL) through inhalation by paddling. Meth or DW vapor was delivered in two seconds as soon as the light cue, placed directly over the drug-paired lever, was illuminated. Each drop was followed by a two-second timeout, when the light remained off and lever pressing had no consequence. The number of pedals each animal pressed for inhalation increased daily. The maximum drug use was set at 1 cc daily and the animals did not receive higher amounts of drugs. For two weeks (10), the addicted group was placed in the rodent self-administration modeling apparatus for meth intake for 15 min each day.
3.6. Plasma Meth Measurement
To measure the plasma level of meth, 0.3 - 0.5 cc of blood was collected from the tail vein after two weeks of addiction and the serum was separated via centrifugation (for 10 min at 4000 g) and frozen. The meth level was assayed using gas chromatography-mass spectrometry (GC-MS) (16).
3.7. Behavioral Tests
3.7.1. Behavioral Test One: Locomotor Activity
The motor activity test was performed using an open field box. On the 14th day of addiction, the animals were placed in the test box 30 min after inhalation of meth. The apparatus was made of Plexiglass (100 × 100 × 40 cm), the bottom of which was uniformly black and divided into 16 equal squares in the data registration software. All the tests were performed under similar conditions in terms of temperature, humidity, and time. At the beginning of each test, the rats were individually placed at the center of the box, and their motor activities, i.e., the number of crossings through squares in the center and open-box environment, the time spent in the center or environment, and the distance traveled in 5 min, were recorded by a camera mounted above the apparatus (17).
3.7.2. Behavioral Test Two: CPP Test
CPP test: The CCP apparatus used in this study was composed of three parts. Parts two and three had similar dimensions (30 × 30 × 40 cm). The only difference between these parts was that part two had a black floor, black and white striped walls, and a yellow lamp, while part three had a black wall, a black floor, and a red lamp. Part one was smaller than the other two parts (30 × 15 × 40 cm). It constituted the entrance of the apparatus where the animal was placed to initiate the test; there were guillotine doors between these parts. The CPP test had a five-day pattern, including three separate periods, namely familiarization, conditioning, and test periods.
Familiarization or preference determination: On the first day (before addiction modeling), each animal was placed in the apparatus for 15 min when the guillotine valve was open and the animal could move freely in the apparatus. Simultaneously, the time spent in parts two and three was recorded.
Conditioning period: From the second to the fourth day, the animals received meth and DW twice a day according to the scheduled program. At 8 a.m. on the first day of addiction onset, the rats received meth through inhalation and were placed in the room with lower preference in the previous stage for 45 min. Six hours later, after the consumption of DW in the self-administration apparatus, the animals were again placed in the front room for 45 min with closed guillotine doors. On the second day, a schedule opposite to that applied on the first day was used. The rats first received DW, and six hours later, used meth through inhalation. The third day of conditioning was similar to the first day.
Test period: The first CPP test was performed on the fifth day while the guillotine doors were open. The animal was placed in part one of the apparatus and allowed to move freely for 15 min in all compartments of the apparatus. Simultaneously, the amount of time the rats spent in rooms two and three was measured (18). Changes in preference were determined by comparison of time spent on the test day in the drug-administration room and the time spent in the chamber during the preconditioning phase; measurements were expressed as mean ± SEM.
3.8. Statistical Analysis
All data were plotted and analyzed using GraphPad Prism. Data are presented as mean ± SEM. Statistical significance was assessed using paired or unpaired t test, as appropriate. P values of less than 0.05 were considered statistically significant.
4. Results
4.1. Measurement of Serum Meth Level
The level of meth was determined in the meth-addiction group (n = 7). After two weeks of addiction, blood samples were collected from animals 30 min (19) after the inhalation of meth (2 mg/cc of water). The serum meth levels were measured by GC-MS. The mean serum meth concentration in rats with meth self-administration was 1.92 ± 0.3 ng/mL. The serum level of meth in the current study was in agreement with the behavioral results.
4.2. Behavioral Test One: Effects of Meth Self-Administration Through Inhalation on Locomotor Activity
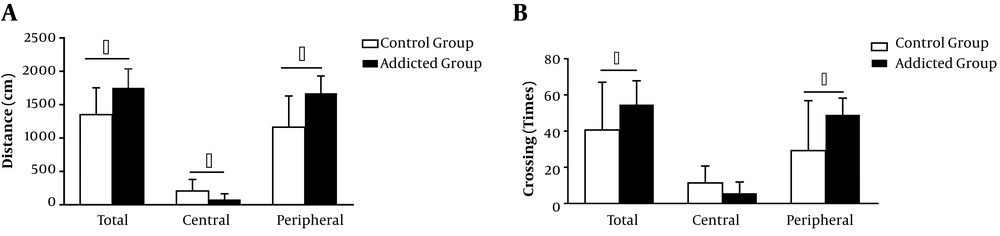
A prominent feature of chronic meth intake in rodents is an increase in locomotor activity, which occurs as a consequence of meth-induced neurotoxicity in the dopamine system of the brain (20, 21). In our study, Wistar rats (n = 7) were administered with inhalable meth for 14 days, and locomotor activity was assessed with the open field test. We found that the inhalation of meth significantly increased locomotor activity in all addicted rats, compared to the baseline or the control group.
Total distance (1753 ± 107.4 cm; P < 0.05) and peripheral distance (1673 ± 97.28 cm; P < 0.05) were longer in the meth group than in the control group (total distance, 1352 ± 142.2 cm; peripheral distance, 1164 ± 164.9 cm; P < 0.05). The numbers of total crossings and peripheral crossings in the meth-addiction group (total crossings, 54.71 ± 4.97; peripheral crossings, 49 ± 3.49; P < 0.05) were higher than those of the control group (total crossing, 40.63 ± 9.34; peripheral crossing, 29.25 ± 9.74; P < 0.05). The data presented in Figure 2 indicate the mean ± SEM of distance traveled and crossings on the 5-min and 30-min post-dose tests.
Effects of meth inhalation (1 - 2 mg/cc) on locomotor activity in the open field during 14 days of self-administration. (A) Total distance traveled (cm), (B) number of crossings, and preference of the peripheral part increased in the meth-addiction group (meth-addiction group n = 7; control group n = 8). Data are shown as mean ± SEM (*P < 0.05).
4.3. Behavioral Test Two: Effects of Meth Self-Administration Through Inhalation on CCP Test
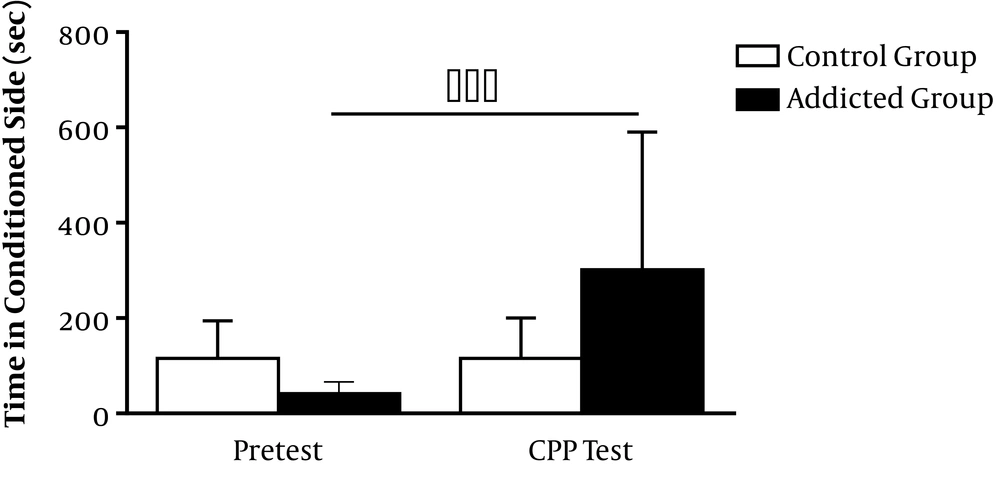
Meth-induced CPP revealed biased responses, as the rats favored the meth-paired environment over the vehicle-paired environment in a classical conditioning procedure. Meth-induced dopamine release in the nucleus accumbens was considered necessary and sufficient for meth-induced CPP (22). After three days of training, meth-addicted rats developed significant CPP for meth inhalation (pretest, 41.43 ± 9.44 s; CPP test, 301.9 ± 109 s; P < 0.001). Figure 3 shows a significant increase in the amount of time spent in the meth-paired environment in the posttest compared to the pretest (P < 0.001).
5. Discussion
The main purpose of this study was to develop a model similar to human inhalant addiction since, despite the high prevalence of this drug among humans, a proper model has not been yet established in laboratory animals. The administration route and dose are of great importance, as neurochemical and behavioral responses to drugs are associated with the rate of increase in drug dosage and maximum concentration (23).
Differences in pharmacokinetics between the routes of administration increase in meth bioavailability and effects. Meth absorption into the bloodstream was found to be slower in subcutaneous dosing than in the intraperitoneal route. Although intraperitoneal dosing of meth is rapid compared to subcutaneous dosing, the hepatic first-pass effect limits the absorbed meth dose. In addition, the overall pattern of meth effect on locomotor activity is related to the administration route and dose, which influence the serum concentration and time of behavioral effects. For instance, a significantly longer duration of traveled distance was reported in subcutaneous meth administration than in intravenous and intraperitoneal dosing (11). Although most animal studies use passive and extravascular injection (e.g. i.p. or s.c.), these routes are not reported among human drug abusers (24).
One of our goals was to manufacture an apparatus, which can be used with a non-invasive technique in animal studies and neuroscience research to reduce the stress of surgery and injection in laboratory animals and to increase the validity of animal experiments. The lack of pain, fear, and stress induction in animals can lead to non-interference of hormones with the test (a standardized test) and the production of more accurate results (25).
Several studies have demonstrated the effects of stress on the metabolism of xenobiotics (26). Stress hormone release, which was increased by approximately 50% or more, could change the neurochemical, physiological, and psychological status of animals and affect their responses to drug treatment (27). Therefore, the removal of a major stress-related factor, e.g., injection, can positively influence the experimental findings.
According to substantial evidence, Corticotropin-Releasing Factor (CRF) is a major factor in emotional dysregulation associated with drug dependence due to the activation of the Hypothalamic-Pituitary-Adrenal (HPA) axis and brain stress systems in the extended amygdala (28). Repeated neonatal maternal separation (an early life stress model in rodents) enhanced locomotor activity and stereotyped responses to meth at relatively low doses (29). Therefore, the elimination of stressors in animals is essential in addiction studies.
According to an established model of surgical stress, this type of stress can induce depression, anxiety-like behaviors, and oxidative stress in the brain (30). Preclinical studies have shown that stress can reinstate nicotine, cocaine, heroin, meth, and ethanol self-administration (31). Therefore, the impact of stress is important on experimental outcomes.
In contrast to traditional studies, mental and physical stress can improve drug use acquisition and increase the risk of addictive behaviors. According to a study by Seo et al., repeated restraint stress weakened the acquisition of meth-induced CCP in the meth conditioning phase (32). In a study by Stuart and Robinson, strategies avoiding restraint could reduce major stress and improve the welfare of laboratory animals via refinement. These animals were in a more positive affective state and had lower levels of stress hormones and aversive behaviors. The injection procedure itself showed little or no aversion, particularly in well-handled animals, and restraint was the major stressor used for the injection (27).
In the development of this modified method, we dissociated between the effects of injection-related discomfort and physical restraint-associated stress and reduced stress associated with routine injections. In this non-invasive method, substances are administered with minimum stress effects. In this model, by pressing the active lever, the animal recognizes the drug as it enters its cage in the form of vapor and inhales it. It seems that drug abuse and addiction can be established in laboratory animals using this method. However, because of the failure to observe the symptoms of tolerance and drug-seeking behaviors, which are the important symptoms of human addiction, it is necessary to adapt these models for human addiction.
Indeed, developing animal models should be more in line with the characteristics of human diseases. Therefore, the best way to develop animal models of addiction is self-administration, through which the animal itself uses the drug. Through time, with the progression of addiction, its dosage increases according to its needs due to tolerance. In this method, addiction and recurrence-related behaviors are investigated. The intracranial and intravenous self-administration methods are very difficult techniques and require fixation of the cannula and catheter on top of the animal’s head and in the internal jugular vein behind the neck, respectively. The main advantage of non-invasive self-administration is that it does not involve any surgical procedures, thereby reducing the possibility of inflammatory responses. This finding is of great importance, as inflammatory responses are potential therapeutic targets and neuroimmune activation is involved in physiological responses to drug intake (33).
The inhalation route of meth self-administration is related to the increased CPP and locomotor physical activity, similar to observations in animals exposed to meth via injection, oral intake, and imposed inhalation (34-37). The present study is in agreement with earlier research, which revealed that meth is a psychomotor stimulant that increases locomotor activity when administered at low doses (38). Similarly, Hall et al. (21) showed that meth increased locomotor activity in the open field. According to a review study by Prut and Belzung (39), the anxiogenic effect of meth is indicated by a reduction in the time spent in the central part of the open field or crossing the central part of the apparatus.
The present results, together with previous findings, indicate the development of CCP following repeated administration of meth (40). As reported in various studies, the potential of meth to induce reinstatement of drug reward-related behaviors is related to its capacity for the activation of the mesolimbic dopamine system. The enhancement of dopaminergic neurotransmission results in glutamate (Glu) release in the prefrontal cortex. Accordingly, the increase in Glu is attributed to meth-induced reinstatement. The medial prefrontal cortex, which is part of the mesocorticolimbic system, facilitates major glutamatergic projection to the nucleus accumbens and ventral tegmental area to regulate dopamine release and simultaneously receive dopaminergic inputs from the ventral tegmental area and nucleus accumbens, which are crucial for the rewarding effect of meth (41).
Moderate and low meth doses may increase locomotion, whereas high doses lead to numerous locomotor activation phases. The induced increase in locomotion is attributed to the dopaminergic mechanism. Different subtypes of dopamine receptors may be involved in receptor activation and consequently multiphasic locomotor activation in response to high meth doses. For inducing the optimal effects of drugs, such as meth, simultaneous activation of dopamine receptors (D1 and D2) is necessary.
Acetylated cyclase is activated, as dopamine binds to the D1 receptor. Subsequently, the resulting cyclic adenosine monophosphate (cAMP) stimulates protein kinase A enzyme and triggers post-synaptic neurons to improve excitability. On the other hand, adenylate cyclase activity is inhibited by stimulating the membrane-bound D2 receptor. Subsequently, the level of intracellular cAMP and postsynaptic neuron excitability can decrease. The mesolimbic brain region is speculated to regulate the response to low doses of meth, while the nigrostriatal pathway can mediate the response to high postsynaptic neuron excitability (42).
In addition to behavioral tests mentioned above, the serum level of meth confirmed inhalation addiction. Higher peak levels of the drug in the plasma are associated with intraperitoneal administration (43), whereas the nasal route of administration has great effects on the brain in promoting behavioral activation despite the low level of meth in the blood (44).
We can conclude that meth inhalation is an effective self-administration route. It presents a promising approach to examine neural and behavioral outcomes in a noninvasive protocol. It is necessary to determine the effects of inhalation self-administration on meth-related complications, such as the brain and respiratory disorders.
5.1. Recommendations for Future Studies
The analysis of plasma corticosterone concentrations and comparison with other methods of drug self-administration are suggested for future studies.