1. Background
Type 2 diabetes mellitus (DM) is increasing worldwide, and the disease, as a significant public health problem, has been found in 22.0% of individuals aged 60 years and over in Iran (1). Diabetic patients are at high risk for vascular damages leading to nephropathy, retinopathy, neuropathy, and cardiovascular and cerebrovascular diseases. Previous studies suggest that cognitive impairment is another complication that the elderly with diabetes may encounter (2-9). However, the American diabetes association (ADA) has not included cognitive disorder in its 2015 treatment guidelines as an important complication of type 2 diabetes (10).
Although risk factors associated with the harmful effects of type 2 diabetes on accelerated cognitive aging need to be more researched, cerebral microvascular damages might have a significant part to play (11-13). Some risk factors for dementia and cognitive decline in patients with diabetes are duration of disease, hyperglycemia, insulin therapy, and peripheral arterial disease (14-17). Cognitive dysfunction in type 2 diabetic patients may be due to the interaction of metabolic abnormalities related to diabetes (hyperglycemia and hyperinsulinemia), diabetes-specific complications (retinopathy, nephropathy, and neuropathy), and other diabetes-related disorders (ischemic heart disease, cerebrovascular disease, hypertension, low serum HDL cholesterol, central obesity, and depression) (18, 19). In addition, other processes such as advanced glycation end product accumulation or changes in cerebral insulin signaling may promote Alzheimer’s disease (20, 21).
Impairment in cognitive functions, such as planning, organizing, insight and attention, is found in patients with diabetes (22-26). Considering the importance of self-management behaviors in the treatment of diabetes and the high complexity of treatment regimens (eg, blood glucose testing, meal planning, and prevention of acute conditions associated with diabetes treatment such as hypoglycemia and treatment compliance), diabetic patients with cognitive dysfunction may experience significant difficulty in managing their already complicated disease.
Furthermore, identifying diabetes as a modifiable risk factor can help delay the occurrence of dementia (5). Early detection and proper intervention for dementia is consistent with high-quality health care goals and can significantly improve the well- being of both those with dementia and family members who are involved in providing care (27).
2. Objectives
This study aimed at examining the association between specific measures of cognitive function and the glycemic condition of the elderly who participated in the longitudinal study of Amirkola health and ageing project (AHAP) in Babol, Iran (28).
3. Materials and Methods
This analytical cross-sectional study was conducted on elderly participants of AHAP study (28) in Amirkola, Babol, Northern Iran, during 2014 and 2015. In AHAP study (28), all 2234 individuals aged 60 years and over who were living in Amirkola were invited to participate in the study. Of them, 1616 elders participated in the study (response rate: 72.3%). All 1616 senior citizens who were present in the two health centers of Amirkola were invited to participate in the stud by phone or home visits. Data on sociodemographic characteristics, history of other chronic diseases (eg, myocardial infarction or angina pectoris), and diabetes- specific measures, such as medications, were collected using a questionnaire. Each assessment round consisted of a physical examination including height, weight, and blood pressure measurements, which was performed by the educated staff at the research center. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). Blood pressure was measured with the participant in the supine position using a calibrated sphygmomanometer (29). Total and HDL cholesterol were measured using standardized enzymatic methods. The history of myocardial infarction or angina pectoris was defined as a self-reported physician’s diagnosis.
Fasting early morning venous blood samples were collected to assess fasting blood sugar (FBS), total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, and triglycerides (TG) levels. Patients with diabetes were defined based on a self-report confirmed by the general practitioner, self-report alone when no general practitioner verification was available, and by diabetic medication (hypoglycemic medications and/or insulin); however, no self-report of diabetes, no use of diabetes medication, and two fasting blood glucose levels more than or equal to 126 mg/dL were also considered (30, 31).To consider other factors that might have moderated the association between diabetes and cognitive function, we examined the effects of other drugs that were assessed from the pharmaceutical blisters or vials of the participants.
Mini-mental state examination (MMSE) was used to assess cognitive function. This test is a commonly used and reliable scale for assessing the cognitive level of an individual (32). This test is quick to administer at bedside (33-35) and assesses several aspects of cognition using specific questions related to attention, orientation, memory, calculation, and language. Those patients whose score was less than 25/30 are considered to be having cognitive impairment (34). MMSE includes specific questions, and scoring is based on 30 total points, with the scores of 25 to 30 as cognitive impairment, 20 to 25 as mild, 10 to 20 as moderate, and 0 to 10 as severe cognitive impairment. The reliability and validity of the Persian version of this test was examined, showing a valid and reliable measure for screening cognitive impairment in elderly Iranians, and it has overall sensitivity and specificity of 98% and 100%, respectively (36).
The 15-item geriatric depression scale (GDS) was used to assess depressive symptoms. It is a well-validated tool often used to screen depressive symptoms in older individuals (37). This measure is scored based on a 15-point scale and impairment is indicated by a score of 6 or higher (37-39). Its reliability and validity have been assessed in elderly Iranians and it proved to be a proper screening instrument for major depression evaluation in the elderly in Iran (38).
Patients with severe visual impairment, who were unable to complete the tests, were excluded from the study.
For statistical analysis, demographic and health- related characteristics were compared between the two diabetic and non-diabetic groups. T test, chi-square, ANOVA, and logistic regression tests were used for data analysis. All analyses were performed using SPSS software (Version 16).
This research was approved by the ethics committee of Babol University of Medical Sciences (ethical committee approval code: MUBABOL.REC.1392.4).
4. Results
Out of 1616 elderly individuals (≥ 60 years old), 1,503 were enrolled in our study; of them, 835 were male (55.6%) and 668 female (44.4%). The mean age of the participants was 69.07 ± 7.28 (minimum 60 and maximum age 92) years. Of them, 95 (6.3%) were unemployed, 589 (39.2%) housekeepers, 478 (31.8%) employed - except housekeeping-, 331 (22.0%) retired, and occupation was not mentioned in 8 cases.
Of the participants, 465 (31.9%) had diabetes and 1038 (69.1%) were normoglycemic (non-diabetic). Their general baseline characteristics are demonstrated in Tables 1 and 2. There were significant differences in weight, BMI, systolic blood pressure, serum TG, LDL, and past medical history of MI or angina pectoris between diabetic and non-diabetic patients (P value < 0.05).
| Variable | Mean ± Standard Deviation Values | P Value | |
|---|---|---|---|
| With Diabetes | Without Diabetes | ||
| Age, y | 68.33 ± 6.941 | 69.40 ± 7.409 | 0.008 |
| Height, m | 157.340 ± 8.81 | 157.500 ± 9.41 | 0.755 |
| Weight, kg | 69.565 ± 12.73 | 66.744 ± 12.87 | < 0.001 |
| BMI, kg/m2 | 28.07 ± 4.51 | 26.78 ± 4.51 | < 0.001 |
| Systolic blood pressure, mmHg | 145.79 ± 22.99 | 141.32 ± 21.62 | < 0.001 |
| Diastolic blood pressure, mmHg | 82.17 ± 12.22 | 81.15 ± 11.66 | 0.122 |
| Fasting blood sugar, mg/dL | 163.70 ± 59.68 | 98.20 ± 11.24 | < 0.001 |
| Serum total cholesterol | 196.03 ± 46.57 | 197.47 ± 39.57 | 0.564 |
| TG, mg/dL | 182.69 ± 96.35 | 150.60 ± 76.52 | < 0.001 |
| HDL, mg/dL | 38.83 ± 4.66 | 38.68 ± 4.25 | 0.535 |
| LDL, mg/dL | 122.32 ± 39.91 | 128.65 ± 34.55 | 0.004 |
| MMSE score | 25.02 ± 3.86 | 25.51 ± 3.40 | 0.018 |
| Variable/Groups | No. (%) | P Value | |
|---|---|---|---|
| With Diabetes | Without Diabetes | ||
| Gender | < 0.001 | ||
| Male | 47.5 | 59.2 | |
| Female | 52.5 | 40.8 | |
| Age | 0.168 | ||
| 60 - 64 | 39.1 | 35.8 | |
| 65 - 69 | 23.2 | 19.8 | |
| 70 - 74 | 15.5 | 18.6 | |
| 75 - 79 | 14.8 | 15.4 | |
| 80 - 84 | 5.2 | 6.8 | |
| ≥ 85 | 2.2 | 3.5 | |
| Marital status | 0.134 | ||
| Married | 82.4 | 86.9 | |
| Widow | 11.8 | 9.2 | |
| Widower | 5.6 | 0.2 | |
| Divorced | 5.6 | 3.8 | |
| Educational level | 0.34 | ||
| Illiterate | 65.6 | 62.7 | |
| Primary and secondary school | 26.9 | 30.5 | |
| High school and University | 7.5 | 6.7 | |
| Occupation | < 0.001 | ||
| Unemployed | 8.8 | 5.2 | |
| Housekeeper | 45.8 | 36.4 | |
| Retired | 20.2 | 22.8 | |
| Occupying (except housekeeping) | 24.7 | 35 | |
| Unknown | 0.4 | 0.6 | |
| Glucose lowering drugs | < 0.001 | ||
| Yes | 54.8 | 0.4 | |
| No | 45.2 | 99.6 | |
| Past history of Myocardial Infarction | < 0.001 | ||
| Yes | 9 | 3.9 | |
| Past history of angina pectoris | 0.003 | ||
| Yes | 22.2 | 15.6 | |
| Depressed (according to GDS) | < 0.001 | ||
| Yes | 50.9 | 38.7 | |
| MMSE score < 25 | 0.029 | ||
| Yes | 34.6 | 28.9 | |
| Body mass index, kg/m2 | < 0.001 | ||
| < 25 | 21.9 | 37.7 | |
| 25 - 29.99 | 48.8 | 40.3 | |
| ≥ 30 | 29.2 | 22 | |
Of the elderly, 1,042 (69.3%) had normal cognition and 461 (30.7%) showed impaired cognition according to MMSE cut values. Mean, SD, and 95% confidence interval for the mean of MMSE score in the two groups were calculated as 25.51 ± 3.40 (25.30 - 25.72) and 25.02 ± 3.86 (24.63 - 25.46), respectively (P = 0.018).
The association of cognitive condition with diabetes status is presented in Table 3. Also, we compared the prevalence of cognitive impairment (MMSE score less than 25/30) in the two groups and the results revealed that the prevalence of cognitive impairment was higher in the diabetic group (OR: 1.303, 95% CI: 1.032 - 1.645, P = 0.029).
| Diabetes Status | Normal MMSE | Mild Impaired Cognition | Moderate Impaired Cognition | Severe Impaired Cognition | Total | P Value |
|---|---|---|---|---|---|---|
| Without diabetes | 738 (71.1) | 233 (22.4) | 65 (6.3) | 2 (0.2) | 1038 (100) | 0.048 |
| With diabetes | 304 (65.4) | 121 (26.0) | 36 (7.7) | 4 (0.9) | 465 (100) |
aValues are expressed as No. (%).
All the associated variables that had been assessed (gender, age, educational level, occupational status, BMI, antidiabetic medication, past history of myocardial infarction, past history of angina pectoris, and depression-according to GDS-) were entered into logistic regression analysis. The adjusted odds ratio of cognitive impairment is demonstrated in Table 4.
| Variable | Adjusted OR for Cognitive Impairment (95% CI) | P Value |
|---|---|---|
| With diabetes | 1.359 (0.950 - 1.944) | 0.093 |
| Females | 3.013 (2.128 - 4.267) | < 0.001 |
| Age, y | ||
| 60 - 64 | 1 | < 0.001 |
| 65 - 69 | 1.267 (0.875 - 1.833) | 0.050 |
| 70 - 74 | 1.461 (1.000 - 2.134) | 0.051 |
| 75 - 79 | 3.444 (2.330 - 5.090) | < 0.001 |
| 80 - 84 | 4.401 (2.590 - 7.479) | < 0.001 |
| ≥ 85 | 5.691 (2.796 - 11.581) | < 0.001 |
| Educational level | ||
| Illiterate | 5.598 (4.037 - 7.763) | < 0.001 |
| Occupation | ||
| Unemployed | 1.626 (1.122 - 2.357) | 0.010 |
| Body mass index, kg/m2 | ||
| < 25 | 1 | 0.006 |
| 25 - 29.99 | 0.608 (0.448 - 0.825) | 0.001 |
| > 30 | 0.754 (0.528 - 1.075) | 0.118 |
| Antidiabetic medication use | 0.792 (0.510 - 1.230) | 0.299 |
| Past history of myocardial infarction | 0.942 (0.526 - 1.686) | 0.841 |
| Past history of angina pectoris | 0.777 (0.554 - 1.092) | 0.146 |
| Depressed (according to GDS) | 1.359 (0.950 - 1.944) | 0.093 |
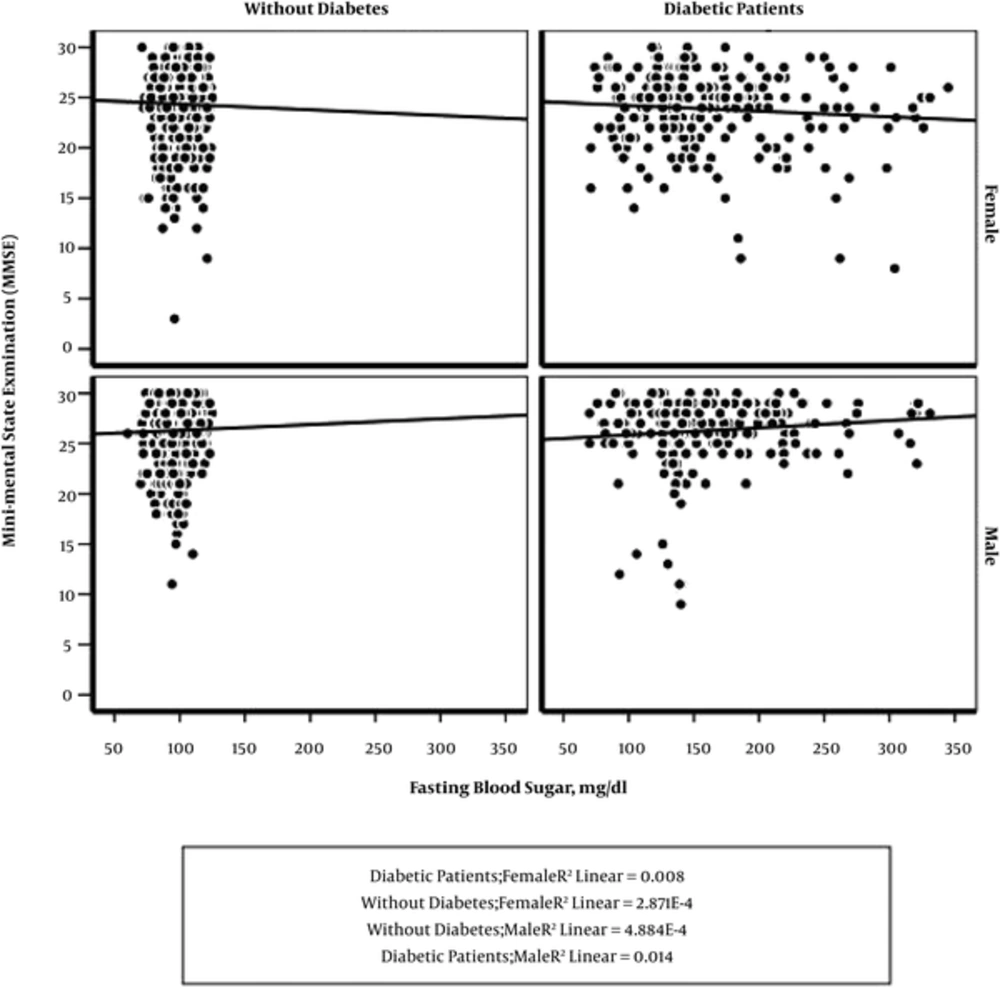
The level of fasting blood glucose had a significant association with MMSE scores, interestingly with differences between the two sexes. When FBS level increased, MMSE score decreased in females, while the MMSE score adversely increased in males (Figure 1). We examined the interaction between gender and diabetes mellitus using two-way ANOVA; no significant interaction was found between sex and diabetes (P = 0.187).
5. Discussion
Our findings revealed that older adults with diabetes mellitus have poorer cognitive functions, and there is a 1.3- fold increased risk of cognitive impairment among people with diabetes. This finding was similar to the research of Van den Berg in which the association of four vascular risk factors (type 2 diabetes, obesity, dyslipidemia, and hypertension) with impaired cognitive functioning was reviewed. They suggested that all the four mentioned risk factors could cause a decline in cognitive functioning, but the association was most consistent in type 2 diabetes and hypertension (40). Furthermore, the statement of Waterloo University declared that type 2 diabetes was associated with poor performance on cognitive tests measuring (41). The study of Logroscino that examined the association of type 2 diabetes with baseline cognitive function and cognitive decline over two years of follow- up in women aged 70 to 81 years, it was found that women with type 2 diabetes had increased odds of poor cognitive function (25% - 35% increased odds of poor baseline score) and substantial cognitive decline (5). The findings of Yaffe study are also compatible with our findings, as they indicated that diabetes mellitus and poor glucose control among diabetic patients are associated with poor cognitive function and greater later decline (6). This higher cognitive impairment in diabetic patients might be correlated to different consequences of diabetes, such as patient’s glycemic condition and micro- and macrovascular damages (19). Body mass index (P value < 0.001), systolic blood pressure (P < 0.001), serum triglyceride level (P < 0.001), and past medical history of MI or angina pectoris (P < 0.05) were significantly higher in diabetic patients than non- diabetic patients.
In our study, higher fasting blood glucose levels were associated with an increased risk of impaired cognition, which is consistent with the results of Crane et al. (42) and Yaffe et al. (6) studies in which they suggested that the severity of diabetes may contribute to accelerated cognitive aging. A wide range of cognitive domains can be impaired in older patients with type 2 diabetes. In the study of Zhou et al. (43), it was mentioned that insulin and its signaling pathway not only regulates glucose metabolism, but also modulates learning and memory. Moreover, in elderly patients with diabetes, it was reported that there is a reduction in cerebral perfusion of the frontotemporal region that plays an important role in memory, judgment, attention, learning ability, and other functions. The study of Ramos-Rodriguez et al. (44) revealed that central vascular disease is related to exacerbated pathology in patients with type 2 diabetes and Alzheimer’s disease.
Older patients with diabetes and concomitant cognitive dysfunction may not be able to follow treatment regimens, such as multiple oral medications, several daily insulin injections, and dietary regimen. These patients can be at increased risk of treatment complications, such as omission of meals leading to hypoglycemia or incorrect dose of drugs. The fact that 17.6% of our study patients who had diabetes did not have a partner is a matter of concern because they may be living alone and the complications associated with their impaired cognitive function can be even more complex.
In our study, patients with diabetes reported depressive symptoms (according to GDS) more than non-diabetic patients (P < 0.001). In a previous study conducted in a similar study population, it was mentioned that the mean of MMSE score was higher in non-depressed elderly (26.0 ± 3.2) compared to depressed ones (24.1 ± 4.5), P < 0.001 (34). Depression in patients with diabetes can increase the health care needs and finances (26); also, self-management of diabetes can be affected by depression (19).
In this study, the level of blood glucose had a significant association with MMSE scores, but this result manifested differently among the two genders: when FBS increased, MMSE scores decreased in females, but the scores increased in males. There was no significant statistical interaction between sex and diabetes mellitus, and this result may be due to the impact of sexual hormones. However, it is highly recommended to conduct studies to investigate the effects of sexual hormones on cognitive function of older adults. In his research, Logroscino has mentioned that type 2 DM affected older women and men disproportionately and suggested further studies to investigate the impact of diabetes on cognition in older women (5). Beauchet indicated that low serum levels of testosterone may be related to cognitive decline in healthy older men (45). Hogervorst reviewed the effects of increasing testosterone on cognitive functions in elderly men and women and concluded that low testosterone levels may predispose to Alzheimer’s disease in men (46). Moreover, Janicki suggested that changes in estrogen levels during ageing may increase the risk of cognitive impairment (47).
The strengths of our study were its community-based sample, its large number of elderly diabetic subjects, and the physical examination and laboratory tests performed to assess the measures that were potentially associated with diabetes and/or cognitive functions.
We evaluated cognitive dysfunctions using MMSE that has been reported to have low sensitivity in detecting subtle cognitive impairment, and this was a limitation of this study. Short physical examination and assessment tools, such as MMSE, have limitations in detecting subtle cognition changes (26). We suggest more sensitive tools to identify subtle cognitive impairments for further studies.
To provide care for the elderly, health care providers and family physicians should pay sufficient attention to identify any cognitive decline in its initial stages in the elderly with impaired fasting blood sugar and determine the association between blood glucose level and cognitive function.