1. Background
Addiction to methamphetamine is now deemed a major worldwide concern (1), an estimated annual global prevalence of 0.4% (2). Iran has a high rate of methamphetamine users insofar as it accounts for 5.2% of the total rate of substance users (3). The long-term use of methamphetamine psychological disorders, in addition to performance-related problems (4), imposes considerable costs on families and societies (5).
Users of methamphetamine, who use it only once, and individuals with underlying mental disorders expedite the incidence of psychosis by 50% to 70% (6) and quitting the patients with methamphetamine dependence that require hospitalization (7).
Methamphetamine-related psychiatric disorders now constitute a formidable challenge to Iran’s health-care system (8), and most patients with methamphetamine dependence are young (9). On a global scale, the emergence of methamphetamine (10) begets serious problems known as “methamphetamine withdrawal symptoms” coming after its sudden withdrawal (11). These symptoms encompass depression, psychosis, and behavioral imbalance (12). The severity of depression after methamphetamine withdrawal cannot be compared with other substances because this kind of depression is so severe that it renders the individuals incapable of performing their daily duties and activities (11). There is a relationship between depression and suicidal ideation after methamphetamine withdrawal (13). Symptoms of methamphetamine withdrawal intensify day by day and may eventually be unbearable and cause relapse (14, 15). Dangerous social injuries such as traffic collisions, rape, and sexually transmitted diseases are other problems that methamphetamine users experience (16). Owing to the recurrence of methamphetamine abuse in early users after withdrawal, it is important to provide a tool for diagnosing withdrawal symptoms in methamphetamine addicts. This study aimed to validate the reliability of the Amphetamine Withdrawal questionnaire version 2 (AWQV2).
2. Objectives
Such methamphetamine-related physical, psychological, behavioral, and social problems call for wide-scope research, and it should be noted that the absence of a valid and reliable tool precludes attempts at diagnosing and treating methamphetamine withdrawal symptoms. The Amphetamine Withdrawal questionnaire is one of the latest tools developed and used in methamphetamine rehabilitation centers (17, 18). Therefore, considering the research and clinical needs as well as the different structure of this questionnaire in different cultures, it was important to investigate the psychometric properties of AWQV2 in Iranian society.
3. Methods
3.1. Participants
The research used a cross-sectional descriptive method. The statistical population comprised all male and female patients with methamphetamine dependence who referred to the addiction recovery centers or were hospitalized in the Psychiatric Ward of Farabi Hospital in Kermanshah, Iran, in 2017. The determination of a minimum sample size required for the collection of structural equation modeling data is crucial in exploratory and confirmatory factor analyses (19). According to Kline, a sample size of 10 to 20 is required for each exploratory factor analysis, but a minimum sample size of 200 is justifiable. In the confirmatory factor and exploratory analysis, the minimum sample size was determined based on factors, not variables. The sample size recommended for the confirmatory factor analysis was approximately 200 for 10 factors (20, 21). In the present study, the sample size was set at 388 individuals with methamphetamine dependence (215 women and 175 men) for the conduct of the exploratory and confirmatory factor analyses. A total of 22 individuals were excluded from the study because they refused to give consent for participation.
Sampling was conducted in two stages. First, five addiction recovery centers were randomly selected using the table of random numbers. Then, from patients with methamphetamine dependence, those who were willing to take part were selected by convenient sampling.
3.2. Procedure
Ethical considerations included respect for the patient, confidentiality of the patient’s data, coordination with the physicians and authorities of the center, and controlling psychotic symptoms. Each participant was verbally provided with information regarding the study and the contents of the information sheet. All the participants signed a consent form in which the study procedures were explained.
We obtained the permission of the author (Dr. Manit Srisurapanont) by email for the translation of the questionnaire into Persian and its subsequent localization. The original version was first translated into Persian. It was then translated into English by two experts in Persian. Then, the original and translated versions were compared, and the translation errors were corrected by two translators. To ensure the equivalence and meaningfulness of both English versions, back-translation with the original version was performed (22). In the final stage, the questionnaire was pre-tested on 20 subjects to identify possible ambiguities in the semantic understanding of the questionnaire.
Then, the validity of the content of the questionnaire was done by interviewing experts in this field.
In the qualitative evaluation of the content validity of grammar compliance, the use of appropriate words emphasized the importance of items in their place, and the questionnaire was presented to 10 experts to determine face validity, and then the difficulty level, the amount of inadequacy, the ambiguity of the expressions, or the failure of the word meanings were corrected, and their comments were applied as minor changes to the questionnaire (23).
It should be explained that this questionnaire is in the form of a research project approved by Kermanshah University of Medical Sciences under the number (grant number: 96414).
3.3. Ethical Considerations
The study was approved by the Vice-chancellor of Research and Technology and the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran, on July 29, 2017 (registration no.: KUMS REC.1396.360).
3.4. Measurements
3.4.1. Amphetamine Withdrawal Questionnaire Version 2
Developed by McGregor et al. (18), the Amphetamine Withdrawal questionnaire version 2 (AWQV2) consists of 10 items scored on a 5-point Likert scale ranging from 0 to 4 (“not at all”, “very little”, “a little”, “quite a lot”, and “very much”). The total score ranges from 0 to 40. This questionnaire comprises three factors of hyperarousal, anxiety, and reversed vegetative symptoms and has an internal consistency (Cronbach’s alpha) of 0.90 and a validity (r) of 0.55 (17, 18).
The AWQV2 is to be completed by the patient 24 hours after quitting (17, 24). In the present study, the questionnaire was completed three to seven days after methamphetamine withdrawal symptoms.
3.4.2. Advanced Warning of Relapse (AWARE) Questionnaire AWAER Questionnaire
This questionnaire was developed in 1882 by Miller et al. and comprises 28 items (14). The questionnaire is scored on a 7-point Likert scale (never = score 1 to always = score 7). The psychometric properties of this desirable instrument and its reliability have been reported 0.90 (15, 16).
3.5. Statistical Analysis
Cronbach’s alpha, Pearson’s correlation coefficient, convergence validity, exploratory factor, χ2, degrees of freedom, root mean square error of approximation, goodness of fit index (GFI) adjusted goodness of fit index, adjusted goodness of fit index (AGFI), comparative fit index (CFI) sign of model fit and normed-fit index (NFI) sign of model fit. Analyses were performed using SPSS software version 25, the LISREL software version 8.7, and IBM AMOS software, version 25.
4. Results
The study population was comprised of 388 patients, 215 women and 173 men, aged between 16 and 74 years (mean = 36.94 ± 10.294) (Table 1).
| Items | Scale Variance with Question Removal | Scale Variance with Question Removal | Correlated Whole Correction | Cronbach’s Alpha by Removing the Question | Min | Max | Mean ± SD |
|---|---|---|---|---|---|---|---|
| Have you been craving amphetamine or methamphetamine? | 20.43 | 40.93 | 0.033 | 0.70 | Total score | ||
| Have you felt sad? | 19.48 | 34.09 | 0.547 | 0.66 | 0.00 | 4.00 | 2.20 ± 0.65 |
| Have you lost interest in things or no longer take pleasure in them? | 19.92 | 36.14 | 0.36 | 0.69 | |||
| Have you felt anxious? | 19.4 | 35.34 | 0.47 | 0.68 | 0.00 | 4.00 | 1.99 ± 0.83 |
| Have you felt as if your movements are slow? | 19.63 | 33.1 | 0.55 | 0.66 | |||
| Have you felt agitated? | 19.53 | 33.57 | 0.55 | 0.66 | 0.00 | 4.00 | 2.30 ± 0.89 |
| Have you felt tired? | 19.2 | 35.52 | 0.50 | 0.67 | |||
| Has your appetite increased, or have you eaten too much? | 20.15 | 38.81 | 0.18 | 0.71 | 0.00 | 4.00 | 2.18 ± 0.79 |
| Have you had any vivid or unpleasant dreams? | 19.91 | 34.82 | 0.43 | 0.68 | |||
| Have you been craving for sleep or sleeping too much? | 19.94 | 38.14 | 0.21 | 0.71 | |||
Internal consistency and Cronbach’s alpha were utilized to assess the reliability of the data based on a 1-time administration of the questionnaire. The results revealed a Cronbach’s alpha of 0.72. Below, a more detailed examination of the descriptive properties of the research instrument is provided.
The mean and scale variance were reported after removing each item (Table 1). Based on the corrected Pearson’s correlation coefficient, the correlation between each score and the total score was high, showing the acceptability of the items. It was also observed that the reliability was decreased or changed minimally by the removal of each item. Moreover, the results of the Kolmogorov-Smirnov test revealed the normality of the distribution of the variables (P > 0.05).
The Kaiser-Meyer-Olkin (KMO) test and the Bartlett test of sphericity were performed. The value of the KMO test was 0.81, denoting a sufficient sample size. Also, the Bartlett test of sphericity yielded a χ2 of 812.38 and a P value of less than 0.001. Therefore, it was possible to perform the factor analysis.
Based on the results obtained from Table 2, it is clear that the three factors have eigenvalue higher than one, and together explain about 58% of the variance of the questionnaire such that factor 1 is 32.64%, factor 2 is 13.82%, and factor 3 is 11.26% (Table 3).
| Items | Questions | Rotational Element Matrix | ||
|---|---|---|---|---|
| Elements | ||||
| 1 | 2 | 3 | ||
| Q7 | Have you felt tired? | 0.762 | ||
| Q6 | Have you felt agitated? | 0.772 | ||
| Q5 | Have you felt as if your movements are slow? | 0.740 | ||
| Q4 | Have you felt anxious? | 0.731 | ||
| Q2 | Have you felt sad? | 0.683 | ||
| Q8 | Has your appetite increased, or have you eaten too much? | 0.799 | ||
| Q10 | Have you been craving for sleep or sleeping too much? | 0.751 | ||
| Q1 | Have you been craving amphetamine or methamphetamine? | 0.831 | ||
| Q3 | Have you lost interest in things or no longer 6. Take pleasure in them? | 0.550 | ||
| Q9 | Have you had any vivid or unpleasant dreams? | 0.319 | ||
| Items | Primary Specials | Total Squared of Extracted Loads | Total Squared of Extracted Loads | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Percentage of Variance | Cumulative Percent | Total | Percentage of Variance | Cumulative Percent | Total | Percentage of Variance | Cumulative Percent | |
| 1 | 3.264 | 32.641 | 32.641 | 3.264 | 32.641 | 32.641 | 3.038 | 30.38 | 30.38 |
| 2 | 1.382 | 13.82 | 46.461 | 1.382 | 13.82 | 46.461 | 1.517 | 15.171 | 45.552 |
| 3 | 1.126 | 11.263 | 57.724 | 1.126 | 11.263 | 57.724 | 1.217 | 12.172 | 57.724 |
| 4 | 0.838 | 8.383 | 66.107 | ||||||
| 5 | 0.766 | 7.66 | 73.767 | ||||||
| 6 | 0.663 | 6.63 | 80.398 | ||||||
| 7 | 0.581 | 5.813 | 86.211 | ||||||
| 8 | 0.526 | 5.259 | 91.471 | ||||||
| 9 | 0.454 | 4.544 | 96.014 | ||||||
| 10 | 0.399 | 3.986 | 100 | ||||||
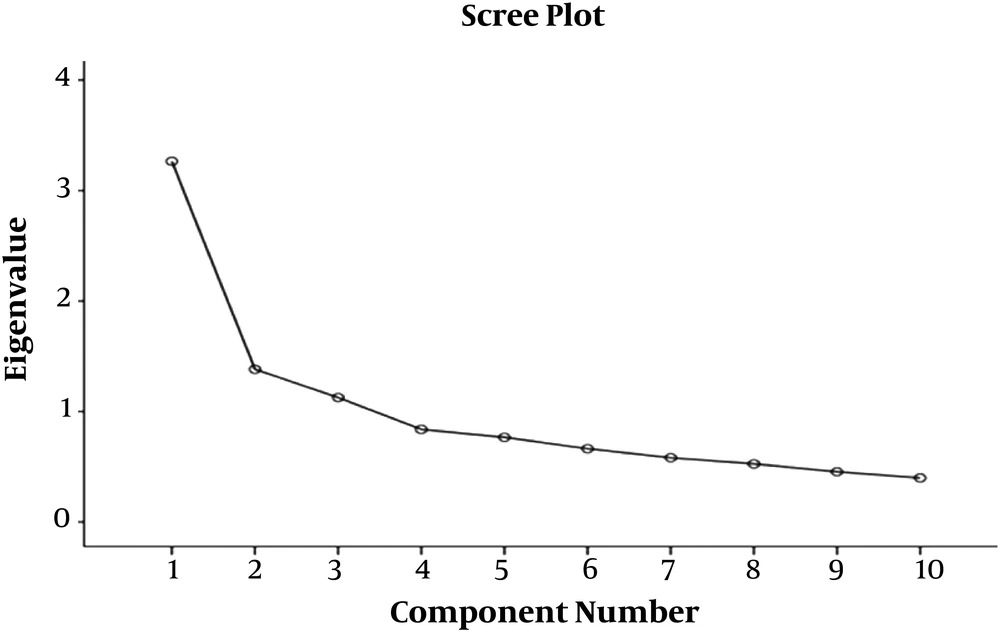
It is clear, based on Table 2, that the three factors had an eigenvalue of above 0.5. These 3 factors explained approximately 58% of the total variance of the questionnaire (32.64, 13.82, and 11.26% for the first, second, and third factors, respectively) (Table 2). The scree plot showed the distinction between these three factors compared with the rest.
It was evident that, except for the third factor, almost all the other factors were placed on a slope.
The scree plots were used to determine the number of factors. For this purpose, given the diagram slope, the factors identified in the diagram steep slope were considered the main factors, and the factors parallel to the slope line axis were avoided. The scree plot contributed to the identification of the three factors as the components of the AWQV2 questionnaire. Accordingly, the scree plot below illustrates the distinction between the three factors relative to the rest and followed by the third factor; the remaining factors are almost on the same slope (Figure 1).
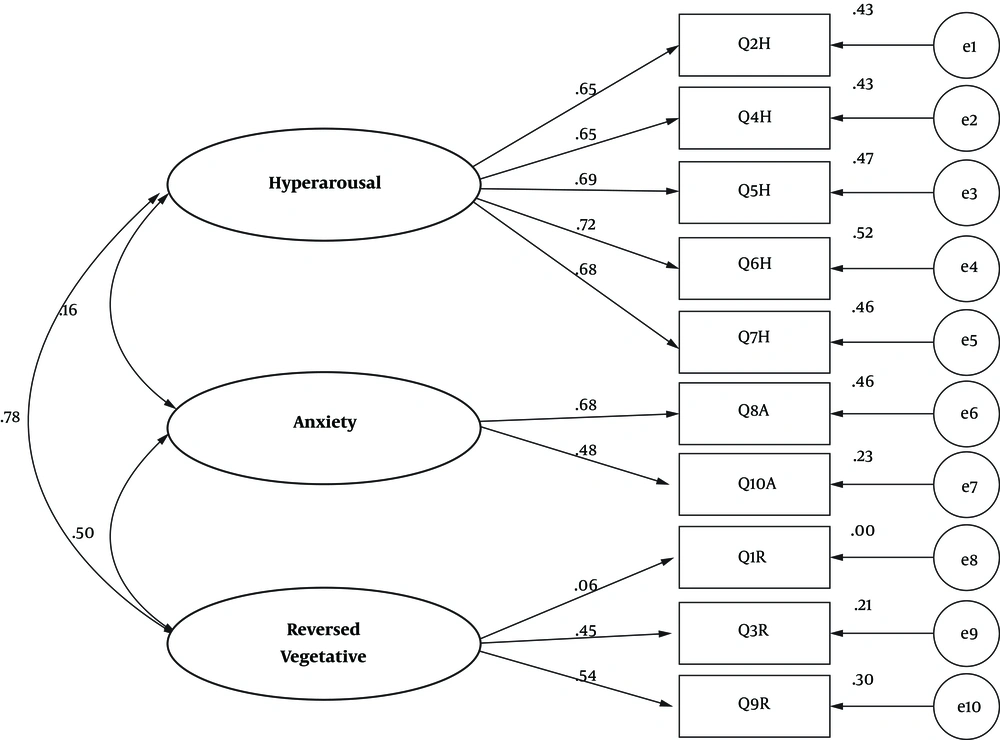
Confirmatory factor analysis and varimax rotation were used to verify the validity of the AWQV2 scale. The confirmatory factor analysis results showed that all items had a significant load factor, greater than 0.319 (the minimum acceptable rate = 0.31) (P = 0.0001), and as explicated in the original study, the items were loaded on the self-loading factors.
Thus, the items 2, 4, 5, 6, 7 were loaded on factor 1 (i.e. reverse vegetative); the items 8 and 10 were loaded on factor 2 (i.e. anxiety); the items 1, 9, 3 were loaded on factor 3 (i.e. hyperarousal). The three factors explained 0.58 of the total variance, and as the general model was properly fitted, all of the items remained on the assumed factors (Figure 2).
Based on the commonly used criteria, a model with a goodness-of-fit index of above 0.9 is an acceptable model. Nevertheless, it determined a cut-off point of 0.95 for goodness-of-fit indices. Root mean square error of approximation of below 0.05, 0.05 to 0.08, 0.08 to 0.1, and above 0.1 shows a good, acceptable, average, and weak fit of the model, respectively (Table 4). Based on the values presented in the table, it is clear that the indices showed a good fit of the model. Therefore, the 3-factor model was confirmed with the confirmatory factor analysis (25).
| Statistical Title | χ2 | χ2/DF | DF | RMSEA | GFI | AGFI | CFI | NFI |
|---|---|---|---|---|---|---|---|---|
| Desired limit | ≤ 0.08 | ≥ 0.9 | ≥ 0.9 | ≥ 0.9 | ≥ 0.9 | |||
| Estimate | 04.04 | 2.93 | 32 | 0.071 | 0.93 | 0.92 | 0.95 | 0.93 |
Our results confirmed the three factors of the hyperarousal subscale score, the anxiety subscale score, and the reversed vegetative subscale score; nevertheless, different numbers of items were found for each factor.
The convergent validity of the questionnaire was assessed by calculating the correlation between its total score and the total score of the AWARE questionnaire. The results of Pearson’s correlation coefficient were significant and positive, showing the good convergent validity of the AWQV2. The reliability of the instrument was examined using internal consistency and test-retest reliability methods. Cronbach’s alpha was equal to 0.72 for the AWQV2, indicating that this instrument had an acceptable internal consistency. Cronbach’s alpha for the subscales of this questionnaire was 0.60 to 0.72. The test-retest reliability was investigated by administering the questionnaire twice at a 2-week interval on 30 patients, and the results were compared with the correlation coefficient of 0.77. The results showed the good test-retest reliability of this questionnaire. In addition, test-retest at a 1-week interval was performed on a small number of patients.
5. Discussion
The present study was conducted to determine the validity and reliability of the Persian version of the Amphetamine Withdrawal Questionnaire (AWQv2) in amphetamine users. The study population consisted of 388 people (215 women and 173 men) aged 36.94 ± 10.25 years (range: 16 - 74 years). The reliability of the AWQV2 was confirmed with a Cronbach’s alpha of 0.72 for the entire scale and 0.6 to 0.72 for its subscales, indicating the good internal consistency of the scale. The test-retest coefficients (0.77) further confirmed the stability of the entire scale and its subscales. These results are in agreement with those reported by Srisurapanont et al. (17) and McGregor et al. (18), who reported good reliability for the questionnaire and good factor analysis of the three subscales, i.e. reversed vegetative, anxiety, and hyperarousal. The factor structure and construct validity of the Persian version of the AWQV2 were assessed in this study using both confirmatory and exploratory factor analyses. Further, factor analysis revealed three factors for Farsi AWQV2, including reversed vegetation, anxiety, and arousal.
Moreover, Pearson’s correlation coefficients between the AWQV2 and AWARE showed an acceptable convergent validity. Proper treatment for drug users mandates a patient assessment and monitoring tool for those who are involved in the treatment of addiction.
Methamphetamine is among the drugs with a high rate of relapse and treatment failure. The various treatments used in this field have a high rate of relapse, which becomes an issue when trying to treat amphetamine users (26).
Overall, with its appropriate reliability and validity coefficients and easy implementation in different situations and groups, the AWQV2 can be used by researchers in various research and clinical fields in methamphetamine rehabilitation centers. Compared to previous studies (17, 18), new statistical methods were used in the present study, including confirmatory factor analysis and convergent validity assessment in LISREL version 8.5. Compared to the present study, previous studies on methamphetamine have studied much smaller sample sizes. Also, very few studies have been conducted on female samples, and studying women becomes more crucial due to the various impacts of this gender on different aspects of life, especially the family, childrearing, and the society as a whole (27).
5.1. Limitations
The major limitation of this study was the lack of access to a larger sample of methamphetamine abusers.
5.2. Recommendations
Future studies are recommended to assess the psychometric properties of the Persian version of the AWQV2 using other methods such as structured interviews and behavior observations.
5.3. Conclusions
Using the ten-question questionnaire of AWQV2, we may adequately measure the characteristics of the withdrawal symptoms of methamphetamine withdrawal in its dependents. Based on the results, the AWQV2 has very good psychometric properties and may be used in research and therapeutic interventions. Therefore, the tool may be used for research purposes and planning for diagnosis, treatment, and reduction of injury.