1. Introduction
Neuroleptic malignant syndrome (NMS) is a rare idiosyncratic disorder caused by an adverse reaction to neuroleptics that occurs in 2% to 3% of patients, who are treated with neuroleptics and has a mortality risk of 10% to 20% (1). This syndrome may lead to death or permanent damages as neurological effects if not diagnosed and treated quickly (2, 3). This reaction has been reported with both first-generation and second-generation antipsychotics. This syndrome is generally characterized by rigidity, tremor, fever, dysregulated hyperactivity of the sympathetic nervous system, mental status change, leukocytosis, and elevated creatinine kinase (CK). However, several case reports and reviews (4-7) suggested that NMS may be induced by second-generation antipsychotics, atypically. Preliminary studies indicated that NMS is induced by second-generation antipsychotics with a lower incidence, milder clinical severity and fatal prognosis, and occurs less than NMSs induced by first-generation antipsychotics (8). Results of a systematic review in 2015 found that even antipsychotics most recently marketed are not free of the risk of inducing NMS. In addition, clozapine, aripiprazole, and amisulpride can create a more atypical view, e.g. they can show extrapyramidal symptoms with less severity or high fever (8). Notably, several cases of NMS induced by clozapine (CLZ-NMS) presented with different clinical features than first generation antipsychotics (FGZ-NMS) have been reported, in which for example there were no cardinal signs. These observations have led to the hypothesis that atypical antipsychotics may create atypical forms of NMS due to their different pharmacological characteristics. Now, while it is commonly accepted that antipsychotics are not free from the risk of inducing NMS, yet, there is still uncertainty concerning the clinical profile of Second generation antipsychotics (SGAs)NMS (9, 10). SGAs are among the most commonly used antipsychotics (11). However, the current information about NMS induced by these factors is limited because intrinsic problems on studying NMS test are under experimental conditions. Thus, case reports are among the main sources of information for clinicians (8). In a systematic report of NMS induced by SGAs, little significant risk factors were identified, such as male gender, confusion, dehydration, delirium, and extra pyramidal symptoms in a study (12) while in another study, factors such as non-white race, the number of anti-psychotic drugs, consumption of aripiprazole and increasing/fluctuant dosing patterns have been mentioned in this regard (13). Furthermore, NMS can be described as a complex dysregulation cascade in multiple neurochemical and neuroendocrine systems that can be potentially placed in the end stage of a hyper-metabolic syndrome (14). The exact pathogenic mechanism underlying NMS is still relatively unknown.
It seems that in fundamental triggering elements, decreased dopaminergic tone in CNS is coincided with a dysregulation of autonomic nervous system, and is determined with the loss of hierarchical integration and control. Functional imbalance continued and maintained by different feed-forward cycles lead to the involvement of a larger number of systems, a progressive damage to muscle tissue, and multi-organ failure (14, 15). Hypo-dopaminergic hypothesis is fundamentally based on the fact that the risk of inducing NMS in parallel is associated with the antipsychotic ability to create extrapyramidal symptoms (EPSs) syndrome and the degree of inhibition of dopamine receptor activity, especially subtype D2, in the nigrostriatal pathway (8, 16). Decreased dopaminergic tone also justifies a sudden test, which occurs in the activity of hypothalamic thermoregulatory system, and in fact induces the next dysregulation in an autonomic response (8, 17). In the recent years, serotonergic receptors with the potential to contribute to the pathophysiology of NMS, especially that induced by SGAs, have received attention. This assumption has been adopted by observing important similarities between NMS and serotonin syndrome in clinical features (18). Recently, a hypothesis has been raised that long-term treatment with SGAs may result in an imbalance in serotonin neurotransmission leading to sensitization to SGAs and other psychotropic drugs (18, 19). This paper reports on an NMS case, in which current views and symptoms that occurred during the course of the disease were rare symptoms that are not usually found in NMS.
2. Case Presentation
The patient was a 43-year-old male with schizoaffective disorder under treatment with clozapine, risperidone and amitriptyline. His problems began five days before hospitalization in the psychosomatic ward with fever, reducing communication with others, not eating, poverty of speech, and drooling. He was admitted to a single specialized university hospital of psychiatry by his family and was referred to infectious emergency in another hospital due to fever with suspected pneumonia after visiting a resident of psychiatry. He was hospitalized at the infectious ward for five days, and psychiatric consultation was requested during hospitalization and discontinuation of clozapine and continuation of risperidone were advised. Pneumonia was rejected via lung spiral computerized-tomography (CT) scan and ceftriaxone was empirically administered for the patient due to a presumptive diagnosis of urinary tract infection. By intensifying agitation and generalized rigidity and lack of patient’s cooperation for treatment, he was transferred to the psychiatric ward again, according to the hospital psychiatrist’s order. In addition, with reference to hypernatremia (sodium: 152 mmol/L), he was transferred to the psychosomatic ward of university general hospital of Sari, Iran, in summer 2016. The patient was hospitalized at the psychiatry ward with a diagnosis of schizoaffective and schizophrenia disorders from 1997 to 2014 for a total of five times. Notably, he had no function during admission intervals. After the last hospitalization, treatment was clozapine (100 mg) twice a day, risperidone (2 mg) three times a day, and trihexyphenidyl (1 mg) three times a day. Since six months before the recent problem, the patient became taciturn with low appetite for two to three days and his communications were declined with others. During the physical examination, the patient was confused and was not conscious of the time, place, and person. Pupils were of normal size and responded to light and both planters were flexor. The patient was agitated and intravenous lines (IV) were taken for him. The temperature was 38.5°C and blood pressure, heart rate, and respiratory rate were 150/90 mmHg, 100/minute, and 20/minute, respectively.
Neuroimaging was normal. Other differential diagnoses, such as meningitis, encephalitis, substance/overdose withdrawal, metabolic disorders, seizure, and heat stroke, were rejected. Besides, serotonin syndrome was rejected because of severe rigidity, lack of hyperreflexia, clonus, and diarrhea. There was also no drug use of serotonin reuptake inhibitors. Levenson’s criteria is widely used for diagnosis of NMS. The patient completed all major and minor items of Levenson’s criteria. Lead pipe muscle rigidity, stupor, fever, and autonomic dysfunction (tachycardia, excessive sweating, and fluctuations in blood pressure), increased levels of creatinine phosphokinase and leukocytosis was in favor of NMS diagnosis. Moreover, serial electrocardiograms showed sinus tachycardia with no acute change. Table 1 shows a summary of performed tests.
| Admission in Infectious Ward | Admission in Psychiatric Ward | First Day of Hospitalization | Third Day of Hospitalization in Psychosomatic Ward | 10th Day | 20th Day | 22th Day | 23th Day | 30th Day | 40th Day | Normal Range | Unit | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 10/1 | 18/6 | 13/1 | 4 – 10 × 103 | THSD/Cu Mm3 | |||||||
| HB | 11/4 | 12/1 | 10/1 | |||||||||
| PH | 123000 | 159000 | 261/000 | 15000 - 40000 | THSD/Cu Mm | |||||||
| CPK | 2700 | 5400 | 7748 | 108 | 534 | 369 | 71 | 24 - 195 | Tu/L | |||
| LDH | 1026 | 800 | 1008 | 715 | 659 | 650 | 633 | 23 - 460 | Tu/L | |||
| CK – MB | 109 | |||||||||||
| Glu | 100 | 138 | 122 | 91 | 90 - 115 | M8/dL | ||||||
| Na+ | 157 | 152 | 146 | 141 | ||||||||
| K+ | 3/9 | 4/6 | 3/1 | 3/2 | ||||||||
| Mg | 2/3 | 2/4 | 1/9 – 2/5 | Mg/gL | ||||||||
| Urea | 93 | 42 | 22 | 31 | 37 | 11 | 13 - 43 | Mg/dL | ||||
| Cr creatinine | 1/6 | 1/2 | 0/9 | 0/9 | 0/9 | 0/9 | 0/6 – 1/4 | Mg/dL | ||||
| SGOT | 150 | |||||||||||
| SGPT | 57 |
Laboratory Tests
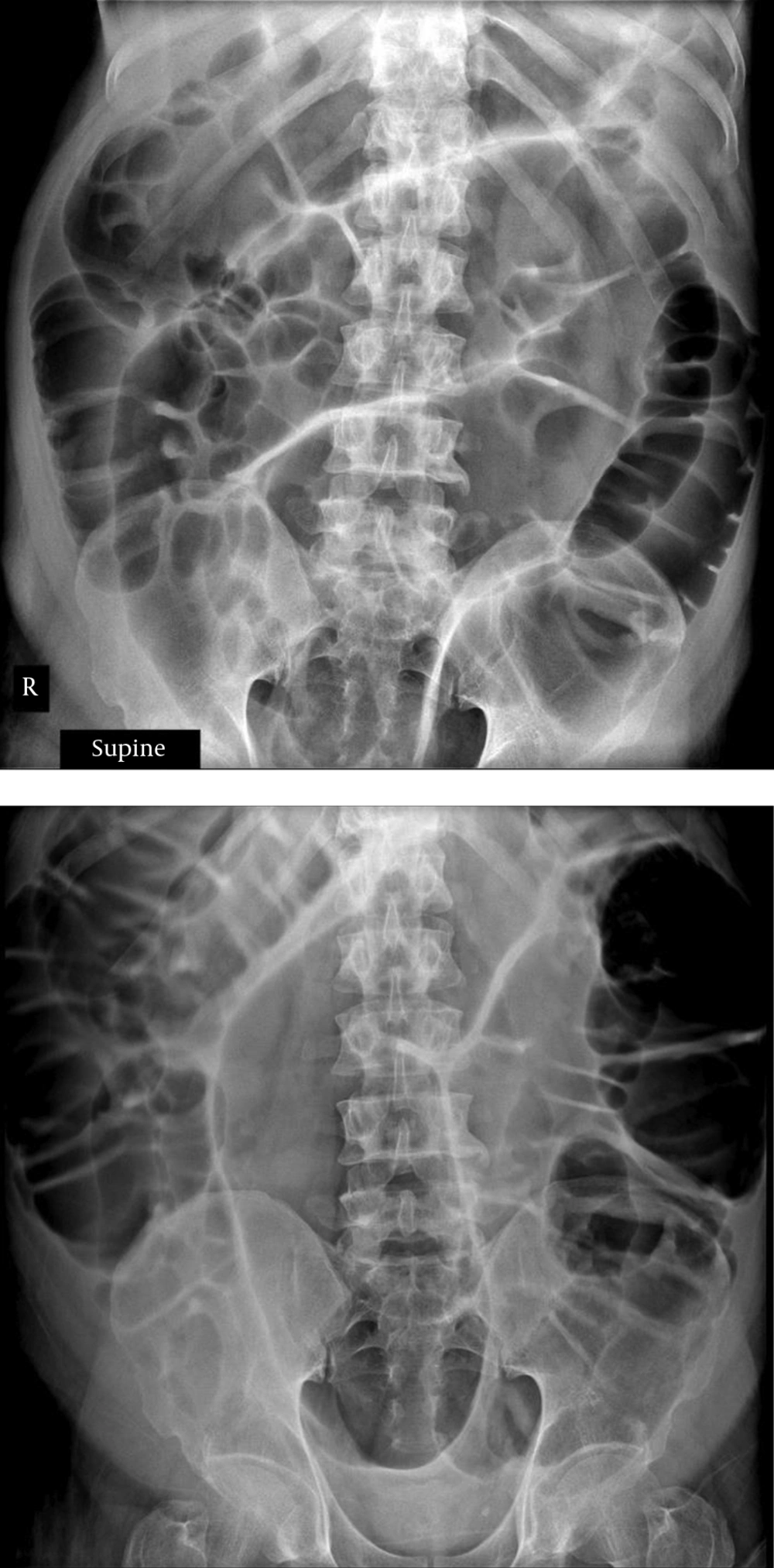
Due to occupied hospital beds at the intensive care unit (ICUs), and negative views of hospitalized patients with major psychiatric disorders in non-psychiatric and psychosomatic wards, the patient was agreed to be in the ICU for only four days during the 42-day hospital stay. Besides, risperidone and clozapine were discontinued. Cold-water sponges were regularly provided to control hyperthermia. Diazepam IV, 30 mg/day, began in three divided doses. In consultation with the oncologist, it was noted that TS equaled 12%, due to anemia, which can be justified according to the patient’s nutritional problems; daily intake of ferrous sulfate tablets was recommended twice per day. Nephrology consultation was requested according to hyponatremia and myoglobinuria. Toxin or drug-induced acute tubular necrosis was raised and proper treatment was started. A total of 12 sessions of electroconvulsive therapy (ECT) for patients during hospitalization and four sessions of ECT after discharge were performed once a week. Due to the lack of intravenous lorazepam in the hospital and after consultation with a nephrologist, lorazepam was administered by intravenous injection as 10 mg, four times a day in the first week of hospitalization. Moreover, diazepam was tapered with starting ECT and was cut after the emergence of ileus with the probability of involvement of benzodiazepines. Six days after admission to the psychosomatic ward, the patient developed a distended abdomen and tense feeling with a mild diffuse abdominal tenderness with no guarding or rebound tenderness or bowel sounds. Marked gaseous distension and abdominal X-ray showed small and large intestines and the liquid-air level were observed in decubitus films (Figure 1). Neomycin consumption began after consultation with the gastrointestinal service, and nasogastric tube was placed and the patient underwent non per oral (NPO) again. With suspicion of benzodiazepine-induced ileus, diazepam was stopped, yet still distention was continued with more intensification. Due to the lack of response to neomycin and GI rest and embedding NG tube, the patient underwent therapeutic sigmoidoscopy for decompression. Distention declined, yet began to increase again after 72 hours, thus the patient was subjected to colonoscopy, and distention was removed. Aripiprazole consumption began with a dose of 2.5 mg per day and was increased up to 5 mg per day. The patient was subjected to electroconvulsive therapy (ECT) once a week; the dose of aripiprazole was gradually increased and valproate sodium was added. Complete recovery occurred at the end of 28 days. Although the patient was psychotic, he showed lack of suicidal and homicidal thoughts and command hallucinations. He was discharged 42 days after hospitalization.
3. Discussion
The aforementioned patient had NMS with simultaneous prescribing of atypical antipsychotics. Diagnosis of NMS is largely based on clinical history and the presence of specific clinical symptoms (14). Unlike the patient introduced here, NMS occurs less in patients on stable dose of antipsychotic drugs for long periods of time or those with appropriate long-term compliance (20, 21). However, antipsychotics polypharmacy increases the risk of NMS (22). A few days later, he showed progressive ileus symptoms. Gastrointestinal disorders in NMS, including sialorrhea, dysphagia, constipation, and fecal incontinence, were reported for this case as well (23, 24). Fluid sequestration in distended intestines, along with excessive sweating, vomiting, loss of fluid intake, and fever may lead to dehydration, which is known as an NMS risk factor (25). The mechanisms underlying the development of ileus in the patient is still speculative, which is undoubtedly a multifactorial and autonomic disorder; hyperthermia, hypoxia, and electrolyte imbalance are all involved in this process (26, 27). Gradually reducing the amount of creatinine kinase from serum appears to be associated with clinical improvement in the patient. It seems that there is no published study on creatinine kinase levels in acute abdomen or pseudo-obstruction. However, it has been shown that the gastrointestinal tube contains only small amounts of creatinine kinase, mainly in the forms of CK-BB isoenzymes (28, 29). Surgical procedures involving gastrointestinal tracts do not increase serum levels of creatinine kinase, and Ck–BB is generally checked after such surgeries (30). The researchers did not check CK isoenzyme patterns in this patient. Late diagnosis led to myoglobinuria and acute tubular necrosis (ATN).
There are possibilities for the origin of paralytic ileus in this patient. One possibility is the existence of anticholinergic side effects of clozapine. However, there were firstly other NMS symptoms and the ileus was created several days after discontinuation of clozapine, this possibility is less likely. In addition, if a hypocholinergic situation is the cause of ileus, sweating should be disturbed as well (31), yet this was not the case in this patient. Another possibility, which is more acceptable, is that the paralytic ileus may be a sign of NMS.
One of the pathophysiologies underlying NMS is hypodopaminergic status in the hypothalamus, which is involved in the regulation of autonomic dysfunction (32). Paralytic ileus can be one of the autonomic dysfunctions in NMS and can be caused by hypodopaminergic status in the hypothalamus (33). Paralytic ileus may be a rare event as one of the symptoms of NMS. Nevertheless, a case report by Tanaka et al. (31) and Lo et al. (32) suggested that this sign should be placed in the list of NMS symptoms. In a patient described in 1993 by Tanaka et al. (31), paralytic ileus existed before the rest of NMS symptoms. In the current patient, similar to the patient reported by Lo et al. (32), in 1989, it was suggested that the ileus appeared after other NMS symptoms. If paralytic ileus was found in patients treated with antipsychotics, strict attention will be needed in terms of the possible development of NMS.
3.1. Conclusions
Clinicians should be aware that NMS could occur due to the use of all antipsychotics, and concomitant use of several antipsychotic medications increases the NMS risk. Psychiatrists should carefully consider this issue. Furthermore, NMS is categorized in differential diagnosis of acute abdomen caused by the pseudo-obstruction. All surgeons should be aware of this possibility when faced with patients under treatment with neuroleptics. High medical doubt, vigilance, and experience are required for diagnosing and beginning the treatment in NMS.