1. Context
After complete thyroidectomy, RRA treatment is an appropriate care option for individuals with differentiated thyroid cancer (DTC) (1). RRA is an effective method of removing residual thyroid tissue following thyroidectomy. In this method, due to the absence of the thyroid, the serum level of thyroglobulin may be an excellent tumor marker. Residual thyroid tissue can cause distant metastases. Today, radioactive iodine is prescribed as an "adjuvant treatment" for ablation. The first report of its use was made in India in 1996. This study reported that I-131 at more than 50 mCi leads to a fixed dose-response curve and, therefore, the use of high doses needs further evaluation (2).
Complete remission in young individuals with disseminated lung micro-metastases may be obtained with RRA treatment. Although complete remission is less common in elderly people, it is nevertheless possible without compromising the life quality (3). Based on American Thyroid Association (ATA), determination of optimal radioiodine dose is difficult, and it seems that ATA recommendations favor low-dose RRA over high-dose RRA. A life-long follow-up should be undertaken to determine the effects of RRA treatment (4).
DTC patients may receive RRA in three situations: residual ablation, adjuvant treatment, and RRA treatment after total thyroidectomy. To obtain negligible serum thyroglobulin (Tg) levels, RRA residual ablation must be performed. Tg is employed as a tumor marker in diagnostic whole-body scans (WBSs) (5). RRA remnant ablation must be done to achieve insignificant serum Tg levels. Tg is a cancer biomarker which is used together with diagnostic whole-body scans (WBSs) (5, 6).
To determine an adequate dose for RRA in low-risk patients with less than 1 cm of tumor and no hazardous characteristics, individuals should be included in RRA treatment (5). The dose of RRA treatment is determined using pathologic features, and a broad range of recommended dosages for RRA has been suggested so far (4, 5, 7). Thyroid cancer prognosis is significantly better than other cancers (8-10).
It seems that employing a higher dosage of RRA leads to higher ablation success rates than utilizing a lower dosage of RRA. In a meta-analysis, Doi and Woodhouse are shown that high-dose RRA is more competent than low-dose RRA for residual ablation (11). The stage of thyroid cancer influences RRA absorption in DTC patients (12, 13). During RRA therapy, less differentiated cells with a low affinity for RRA are retained (3).
At a low dosage (1.1 GBq) according to ATA recommendations, RRA treatment has a high rate of success in low- to intermediate-risk individuals (5). The ablation results of a low-dose RRA (1.1 GBq) for low-risk thyroid cancer patients are as promising as high-dose RRA treatment (3.7 GBq), and there is no need for hospitalization with low-dose RRA (9, 10). Despite current recommendations, more follow-up data is still needed for future studies (4).
It is not possible to evaluate the optimal dose of RRA. In a review performed by Verburg et al., 1298 DTC patients were followed up for 5 years (14). It was shown that elderly low-risk DTC patients who received low-dose RRA (2 GBq) had a more significant DTC-specific mortality and higher recurrence rate than those patients receiving high-dose RRA treatment. Additionally, it was established that elderly high-risk patients receiving low-dose RRA (2 GBq) had a higher rate of DTC-specific death. No differences were seen between low- and higher-dose RRA groups in the overall survival rates (4).
During the RRA residual ablation treatment, notably in patients with 3.7 GBq versus 1.1 GBq, short-term RRA adverse effects, such as nausea, malfunctioning in the neck, tear gland dysfunction, salivary gland dysfunction, and changing feelings, were noted. While most common short-term adverse outcomes are reversible and preventive, some long-term irreversible negative consequences, such as secondary primary malignancy, xerostomia, and infertility, may exacerbate the illness condition (15). In each patient with thyroid cancer, it is essential to examine the implications and benefits of low or high dosage RRA ablation (4).
As a result of limited evidence, several disputes exist about RRA treatment. It is critical to exercise caution while prescribing RRA doses based on patient-specific characteristics. It is crucial to use the optimal dosage of RRA while taking into account the radiation impact on DTC patients. The current recommendations are not supported by sufficient data to allow an individualized DTC patient treatment. The therapeutic and unfavorable effects of RRA treatment should be weighed in light of radiation and patient-specific variables (4).
According to current clinical studies and meta-analyses, the latest ATA recommendations seem to support low-dose RRA. Long-term follow-up information is nevertheless limited, and the effectiveness of RRA therapy has to be taken into account by other factors (4). The present investigation reviews the efficacy of treatment between patients with low and moderate risks and the application of high-dose or low-dose regimens of RRA.
2. Evidence acquisition
2.1. Inclusion and Exclusion Criteria
This investigation included only English-language publications on the dose determination of radioactive iodine used to treat or scan residual thyroid tissue after thyroidectomy surgery. Several studies, for example: (1) published randomized controlled trials (RCTs); (2) systematic reviews and meta-analyses; (3) cohort studies; (4) cross-sectional; and (5) eligible case series, were included in this systematic review covering the last 30 years of research on appropriate dose determination for remnant tissue ablation; however, other studies such as case reports and narrative reviews were excluded.
The main outcome of the treatment was the efficacy determination of ablation on residual thyroid tissue or the determination of residual tumor tissue (undetectable RRA uptake or lack of RRA uptake on scan) or stimulated thyroglobulin level at 2 g/L.
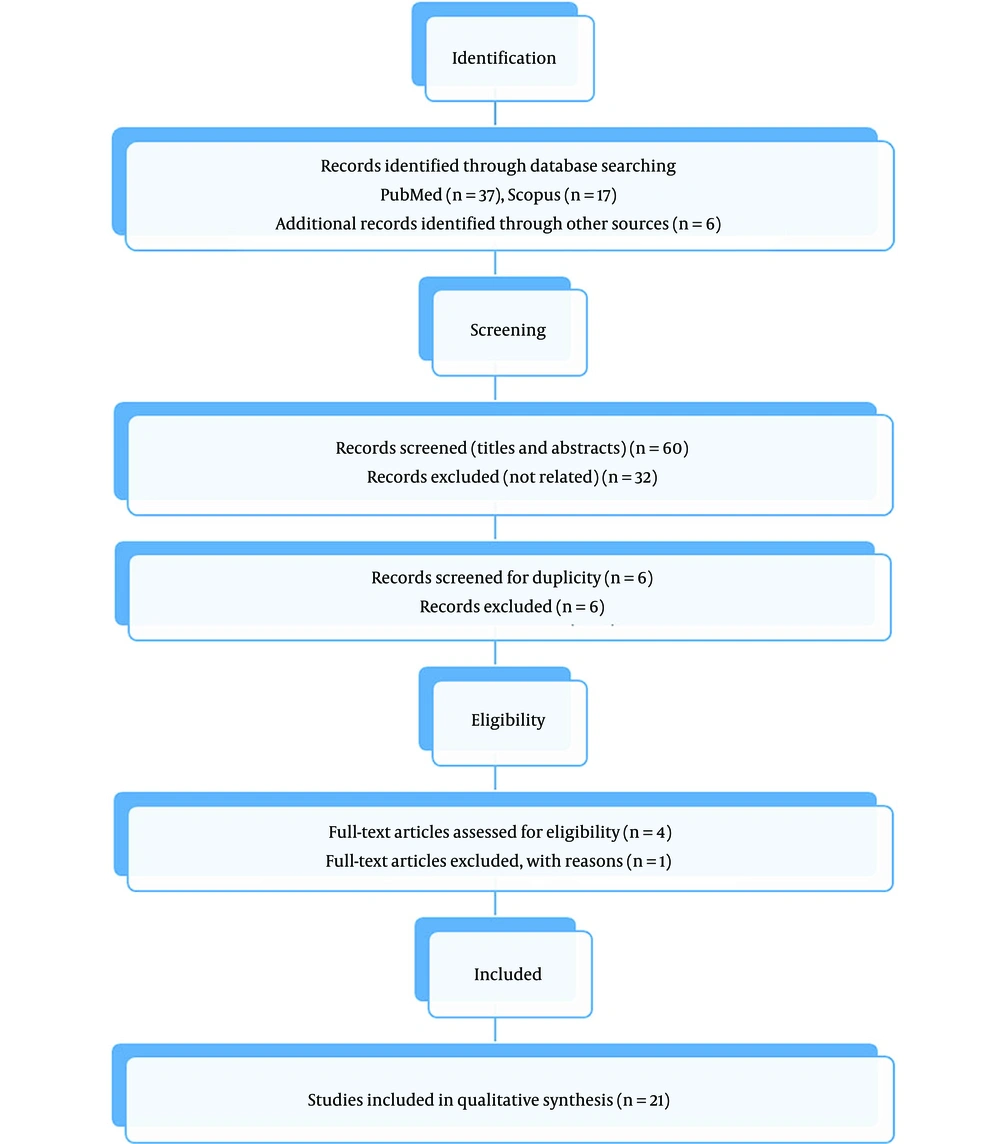
Whole-body scans (pre-and post-RRA), RRA kinetics such as lesion radiation, or long-term thyroid cancer outcomes were secondary outcomes (such as disease recurrence or mortality). In addition, we analyze adverse effects and survival rates. The study's inclusion criteria included the presence of any main or secondary repercussions. After evaluating the study population based on inclusion and exclusion criteria, we selected the most significant or most comprehensive research. Preferred reporting items for the systematic review flow diagram of the study is shown in Figure 1.
2.2. Description of the Search for Relevant Studies
A search of the following electronic databases (no language limitation) was done for possibly relevant citations (April 30, 2021): (since 1990), as well as non-indexed citations in Embase Classic, PubMed, Google Scholar, UpToDate, and Scopus. The computerized search was conducted using text terms and MeSH headings related to thyroid cancer, thyroid surgery, thyroid tissue residual ablation, post-op ablation, radioactive iodine, and RRA, but not hyperthyroidism or nuclear accidents. Due to a lack of translation resources, non-English references were excluded.
2.3. Selection of Studies and Data Abstraction
Two judges independently examined each extracted study. Two separate reviewers evaluated all abstracts or possibly relevant material that had been summarized by the researcher and accompanied by the full text of the publication. Each item that had a summary defect was returned to the researcher by the reviewers to be revised. Each article that was more relevant to our topic was thoroughly assessed by the reviewers.
Each reviewer presented the causes in case of omission of the articles. Finally, both the reviewers and the researcher agreed on the systematic selection and review of the matters. The data were summarized, and the method quality control was evaluated independently by two arbitrators, and then the results were evaluated by a third clinical expert who reviewed and corrected the matters. The quality of the study was evaluated by using the type of study criteria, control group comparison type, results, post-treatment follow-up and statistical methods for the selected studies.
By reviewing the articles in Databases, 47 articles were initially found with similar topics in our review, and finally, 21 articles were eligible for our study (summary information of the articles is given in Tables 1 - 3).
| Author and Year (Reference) | Country | Participants Number | Diagnosis of Patients | Optimal Dose of RRA | Efficacy Percentage | Recurrence or Mortality | Study Objectives |
|---|---|---|---|---|---|---|---|
| Zhang et al., 2015 (16) | China | 102 patients | DTC | - | (84.3%) in the low-dose group and (86.27%) in the high dose | - | Low-dose RAI in DTC with macroscopic extrathyroidal extension and low level of preablative-stimulated thyroglobulin |
| Kim et al., 2011 (17) | Southern Korea | 1024 patients | DTC | 150 mCi | 94.8% | - | Different doses effect of RRA on successful ablation and on long-term recurrences in DTC patients |
| Bal et al., 2004 (18) | India | 565 patients | Papillary thyroid carcinoma and follicular thyroid carcinoma | Between 25 and 50 mCi | 77.6% | - | Radioiodine dose determination for remnant ablation in DTC patients |
| Andersen et al., 2017 (15) | USA | - | DTC | 30 - 50 mCi and doses ≥100 mC | - | 2% | Post- op RRA for DTC Literature Review of Utility, Dose, and Toxicity |
| Dehbi et al., 2019 (19) | UK | 438 patients | DTC | (1.1 GBq) and the standard high dose 3.7 GBq) | - | 1.5% -5.9% | Recurrence after low-dose RRA and rhTSH for DTC (HiLo): long-term results of an open-label, non-inferiority RCT |
| Yoo et al., 2012 (20) | Korea | 161 patients | DTC | 150 mCi | The patients with less strict LID was 80.3% and for very strict was LID 75.6% | - | The success rate of RRA in DTC and comparison between less strict and very strict low iodine diets |
| Jabin et al., 2018 (21) | India | - | DTC | 30 mCi | 54.5% of patients 0 - 30 mCi, 21.5%, 30 - 50 mCi, 10.0% higher doses of 80 - 100 mCi | - | Clinico-social factors to choose RRA dose in DTC patients |
| Jeong et al., 2017 (6) | Korea | 204 patients | DTC | - | 62.5 - 11.25% | - | Low-dose and high-dose postoperative RAI in patients with DTC |
| Samuel and Rajashekharrao, 1994 (22) | USA | 87 patients | DTC | An initial dose rate of 3 Gy/h or more completely ablated up 87.1% | Initial dose rate of 3 Gy/h | - | RRA for DTC: A quantitative dosimetric evaluation for remnant thyroid ablation after surgery |
| M. El-Refaei et al., 2015 (23) | - | 81 patients | Papillary thyroid cancer | 2960 and 3700 MBq | (2960 and 3700 MBq) doses (73% and 77%, respectively) | - | Comparison between low and high dose reablation in PTC patients |
| Iizuka et al., 2019 (24) | USA | 147 patients | DTC | - | (73.5%) low-dose group (70.6%) high-dose | - | Comparison between the DDR iodine ablation prescribed in intermediate-to-high-risk DTC patient |
| Tresoldi et al., 2014 (25) | Italy | 125 patients | DTC | 1,850 MBq | 95.1% in low-risk and 76.2% in intermediate-risk patients | - | 1,850 MBq RAI in association with rhTSH in DTC |
| Wang et al., 2017 (26) | China | 132 patients | DTC | 1100 , 3700 MBq | 86.9% | - | Low activity versus high activity |
| Fallahi et al., 2012 (27) | Iran | 341 patients | DTC | 1110 , 3700 MBq | 41.5 in low dose, and 68.8% in high dose | - | Low versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients DTC patients |
| Kruijff et al., 2013 (28) | USA | 1171 patients | Papillary thyroid carcinoma | - | - | 8% | Decreasing the dose of radioiodine for remnant ablation does not increase structural recurrence rates in PTC patients |
| Patel and Goldfarb, 2013 (29) | USA | 64 patients | DTC | 30 - 100 mCi | 67% | - | Role of post-op RRA in DTC patients |
| Cai et al., 2018 (30) | USA | 515 patients | Papillary thyroid cancer | 30-100 mCi | - | - | - |
| Schlumberger et al., 2018 (31) | France | 726 patients | DTC | 1100 , 3700 MBq | - | - | Outcome after RAI in low-risk thyroid cancer: 5-year follow-up |
| Cherk et al., 2008 (32) | USA | 183 patients | DTC | 1100 , 3700 MBq | 76% - 84% | - | Determination of RAI in thyroiditis and thyroid remnant ablation success rates by 1110 MBq and 3700 MBq post-op ablation |
| Joung et al., 2016 (33) | Korea | 570 patients | DTC | 30 - 100 mCi | 77% - 80% | - | Low-dose and High-dose RRA efficacy With rhTSH |
| Author and Year (Reference) | Study Type | SINGLE OR Multicenter | The Study Duration | Explicit Reporting of Loss to Follow-up | Any Description Statistical Methods |
|---|---|---|---|---|---|
| Zhang et al., 2015 (16) | RCT | Single | 6 months | - | - |
| Kim et al., 2011 (17) | Cohort Retrospective | Single | 6.6 years of median follow-up | - | - |
| Bal et al., 2004 (18) | RCT | Single | Between July 1995 and January 2002, 56 | 59 patients | - |
| Andersen et al., 2017 (15) | RCT | Single | - | - | - |
| Dehbi et al., 2019 (19) | RCT | Multicenter | Jan 16, 2007, until 2017 | - | Kaplan-Meier curves and hazard ratios (HRs |
| Yoo et al., 2012 (20) | RCT | Single | - | - | - |
| Jabin et al., 2018 (21) | RCT | Multicenter | - | - | - |
| Jeong, 2017 (6) | RCT, retrospective | Multicenter | 2003-2006 | - | - |
| Samuel and Rajashekharrao. 1994 (22) | RCT | Single | - | - | - |
| M. El-Refaei et al., 2015 (23) | RCT | Single | - | - | - |
| Lizuka et al., 2019 (24) | Retrospective, RCT | Single | December 2011 and August 2016 | 28 patients | Fisher’s exact test and IPTW |
| Tresoldi et al., 2014 (25) | Retrospective, RCT | Single | - | - | - |
| Wang et al., 2017 (26) | Retrospective, RCT | Single | - | - | - |
| Fallahi et al., 2012 (27) | RCT | Single | 12 months | - | - |
| Kruijff et al., 2013 (28) | Cohort Retrospective | Single | 1990-2012 | - | Multivariate analysis |
| Patel and Goldfarb, 2013 (29) | RCT | Single | 5 years | - | - |
| Cai et al., 2018 (30) | RCT | Single | 5 years | - | - |
| Schlumberger et al., 2018 (31) | RCT | Multicenter | March 28 2007-2015 | - | - |
| Cherk et al., 2008 (32) | RCT | Single | - | - | Logistic regression |
| Joung et al., 2016 (33) | RCT | Single | - | - | Logistic regression |
| Author and Year (Reference) | Goal Daily Intake of Iodine | Lowest Effective Prescribed Dosage | Side Effects Percentage in Patients | Written Instructions for Patients |
|---|---|---|---|---|
| Zhang et al., 2015 (16) | low-dose (1110 MBq) , high-dose (3700 MBq) | 1110 MBq | - | No |
| Kim et al., 2011 (17) | 30 mCi (group A), 80 mCi (group B), and 150 mCi | 30 mCi | 2% of patients developed permanent salivary dysfunction in patients using 150 mCi | Yes |
| Bal et al., 2004 (18) | 25 - 50 mCi | 5 mCi | 2% | Yes |
| Andresen et al., 2017 (15) | 30 - 50 mCi and doses ≥ 100 mC | 30 mCi | 2% | Yes |
| Dehbi et al., 2019 (19) | (1.1 GBq) and the standard high dose (3.7 GBq | 1.1 GB | - | Yes |
| Yoo et al., 2012 (20) | 150 mCi | 150 mCi | - | Yes |
| Jeong et al., 2017 (6) | 124 patients were treated with 3.7 and 5.55 GBq (HD) and 80 patients with 1.11 GBq (LD) in the other center | 1.11 GBq | - | Yes |
| Samuel and Rajashekharrao, 1994 (22) | 0.85 - 9.55 GBq (23 - 258 mCi) | 23 mCi | - | Yes |
| Jabin et al., 2018 (21) | 5 - 70 μSv/h | 5 μSv/h | - | Yes |
| M. El-Refaei et al., 2015 (23) | 2960 MBq and 3700 MBq | 1100 MBq | - | Yes |
| Iizuka et al., 2019 (24) | 1110 MBq , 2960 - 3700 MBq | 1110 MBq | - | No |
| Tresoldi et al., 2014 (25) | 1850 MBq | 1850 MBq | - | No |
| Wang et al., 2017 (26) | 1100 MBq and 3700 MBq | 1100 MBq | - | No |
| Fallahi et al., 2012 (27) | 3700 MBq (170 patients), 1110 MBq (171 patients) | 1110 MBq | - | No |
| Kruijff et al., 2013 (28) | 2.5 GBq (68 mCi) for group A and 4.7 GBq (127 mCi) for group B | 2.5 GBq | - | No |
| Patel and Goldfarb, 2013 (29) | initial high (80 to 100 mCi) or low (less than 30 mCi) | 30mCi | - | No |
| Schlumberger et al., 2018 (31) | (1.1 GBq) and (3.7 GBq) | 1•1 GBq | - | No |
| Cai et al., 2018 (30) | 30 mCi or 50, 100 mCi | 30 mCi | - | No |
| Cherk et al., 2008 (32) | (1110 MBq) and (3700 MBq) | 37 MBq | 21% | NO |
| Joung et al., 2016 (33) | (30 mCi) and (100 mCi) | 30 mCi | - | No |
2.4. Statistical Analyses
Before reaching an agreement on the use of references and content, by statistically evaluating and comparing the content in different references by Kappa, the authors and researchers on the relationship between citation or the review phase (in terms of citations retrieved via electronic search) And insertion in the full text review stage of the discussion. Kappa was calculated at 95% confidence interval using SPSS software (version 26.0, Michigan, USA). For patients with non-metastatic DTC, activity levels of 1.11 and 1.85 GBq are suitable for residual thyroid erosion, with fewer risks of side effects, compared to 3.7 GBq doses, which have completely different benefits.
3. Results
A number of 21 RCTs involving 6699 participants from 1990 to 2021 for this systematic review are analyzed. We compared the patients at low, moderate, and high risk who were treated with various RRA doses. It was discovered that patients treated with high-dose (3700 MBq) and low-dose (1110 MBq) RRAs had a greater success rate in moderate and low-risk patients in most circumstances. Prescription of high and low dosage treatment for patients at moderate and low risk, on the other hand, did not create a meaningful difference (P < 0.05). In patients with low or moderate risk, there were no statistically significant differences in successful ablation rates between low-activity (1.11 - 1.85 GBq) and high-activity (3.7 GBq) radioiodine treatment [odds ratio (OR) 0.80, 95 percent confidence interval (CI) 0.49 - 1.47, P = 0.56].
Whereas, high-dose RRA creates a significant difference to low dose medication patients in terms of the success rate of medication response, when the administration of high-dose for high-risk patients was analyzed [OR 0.97, 95% CI 0.35 - 1.59, P = 0.04)].
Another study found no statistically significant difference between 1.85 and 3.7 GBq for low and intermediate-risk individuals [odds ratio (OR) 0.98, 95 percent confidence interval (CI) 0.20 - 3.7, P = 1]. When other high-risk patients were treated, the same result was obtained [OR 0.83, 95 percent CI 0.15 - 3.98, P = 0.87], and there was no substantial difference in RRA in these patients between the high therapeutic dose (3700 MBq) and the lower therapeutic dose (1850 MBq) (P > 0.05). On the other hand, higher-dose therapy typically resulted in improved treatment results for high-risk people.
In the comparison between the side effects of RRA, the studies on patients with low, medium and high risk demonstrated that the side effects due to RRA in a lower dose of treatments is significantly less than higher doses of treatment. Also, reduced activity of 1.11 GBq substantially reduced side effects such as neck discomfort, gastritis induced by radiation, and salivary dysfunction both during and after ablation [OR 0.63, 95 percent CI 0.42 - 0.93, P = 0.02].
According to data gathered from evaluated RCTs, low activity RRA of 1.115 GBq may be adequate for thyroid tissue residual ablation in patients with low and moderate risk and lead to less common side effects in non-metastasis individuals than high activity RRA of 3.7 GBq, with. Radioiodine therapy with 1.11 GBq showed a substantial advantage in reduced side effects such as neck discomfort, gastritis induced by radiation, and salivary dysfunction both during and after ablation [OR 0.57, 95% CI 0.49 - 0.88, P = 0.03].
4. Discussion
4.1. Overall View
Thyroid remnant ablation (TRA) with radioiodine treatment should be considered first for patients with tumoral lesions. Even though radioiodine has been widely used for decades, choosing an ablative activity is essentially empirical, and no consensus has yet been reached. In candidate patients, determining the proper radioiodine dose for successful ablation is constant (34). The cornerstone of curative-intent therapy is total thyroidectomy, which is followed by frequent RRA of the thyroid remnant to reduce the risk of recurrence (15). After thyroidectomy, radioiodine ablation of thyroid tissue remains the gold standard of therapy for individuals with differentiated thyroid cancer (27). Low-dose radioiodine therapy (RIT) in intermediate- or high-risk DTC patients is still controversial (35). High-dose radioactive iodine is recommended for non-metastatic differentiated thyroid cancer with macroscopic extra-thyroidal expansion (MAEE). It's unclear if these people can be effectively treated with low-dose RRA (16). It's important to remember that RRA therapy with low activity is as effective as RRA therapy with high activity. Some studies suggest that a low dose (LD) of radioiodine (RRA) may be sufficient to treat DTC in people with intermediate risk. On the other hand, these studies looked at the efficacy of RRA therapy independent of the results of the WBS (6). Patients with a low level of pre-ablative stimulated thyroglobulin (ps-Tg) have a better prognosis. However, it is still unclear whether low ps-Tg is more useful in treating low-dose RRA (26).
Remnant ablation may be accomplished via either an empiric fixed dosage or dosimetry-guided procedures. The fact that the majority of the current tools have been adapted for a fixed-dose or standard-dose approach in I-131 remnant ablation poses some technical and logistical challenges. In the late 1970s, low-dose I-131 residual ablation was launched, and several institutions have since validated its efficacy. Although the protocols did not vary considerably, Thyroid Hormone Withdrawal (THW) had a greater success rate than recombinant human thyrotropin (rhTSH). The Tg level at RIT was the only significant independent predictor of effective ablation. The optimal Tg cut-off value for predicting failed ablation was 9 ng/mL (26). At least, individuals having Tg > 9 ng/mL at the time of the first RIT should get a greater dosage of RRA (35). Successful ablation was defined as a negative I-131 whole-body scan and a thyroglobulin (Tg) concentration of less than 2 ng/mL (35). The creation of accurate dosimetry models advances customized treatment toward greater effectiveness and fewer problems. The purpose of this article is to compare the typical maximal empirical activity (250 mCi) in order to maximize therapeutic activity and radioiodine effectiveness in metastatic differentiated thyroid cancer (36).
4.2. Effective Factors for Dosage Determination
In order to achieve the appropriate therapeutic dose in patients undergoing RRA treatment, it is necessary to know a sufficient level of the drug in the patient's body, which will be measured by determining the iodine patient's urine. The appropriate iodine concentration for patients is measured by a urinary iodine test, which indicates a deficiency of the appropriate iodine concentration if it is less than 100 micrograms per liter in patients’ urine, and the proper radioiodine concentration is 100 to 200 micrograms per liter that indicates the appropriate iodine dosage intake by RRA.
Choosing the right dosage involves several factors. Surgeon experience has an important role in surgical success and ablation treatment outcomes (37-39). Compared to low-volume surgeons, high-volume surgeons led to smaller remnant sizes (38), lower complication rates, and shorter hospitalization duration (40).
Surgical methods also affected remnant sizes. Patients who received a single total thyroidectomy had a lower rate of residual uptake than those who received a full thyroidectomy followed by a staging procedure. Postage resulted in more residual uptake than a single total thyroidectomy following total thyroidectomy (38). Additionally, high RRA doses were required in patients who underwent a partial or subtotal thyroidectomy (5).
The residual thyroid tumor and/or thyroid remnant size are two variables that can affect the Tg level after surgery. It is reported in different studies that the recurrence rate depends on postoperative TSH-stimulated Tg (19, 41). Also, ablation failure after 1.1 GBq of RRA treatment was attributed to a high level of postoperative Tg in a study (42). While there is no conclusive evidence, TSH level > 30 mIU/L is conventionally considered appropriate for RRA therapy (5). TSH levels more than 25 mIU/L, according to Fallahi et al., are strongly linked with RRA success (27). Some other studies have not found a significant relationship between serum TSH and RRA success (43, 44). However, the ineffectiveness of TSH levels in ablation cannot be accepted. Age, body area, body mass index (BMI), and creatinine level are associated with increased TSH, and creatinine is a strong index in prediction of RRA success (45). Joung et al.'s 2016 research in Korea found that using rhTSH and thyroid THW was beneficial in preparing patients for RRA (33).
In various studies, a very effective strategy has been devised by the ablation of residual tissue, which is the use of a single injection of rhTSH after a short period of THW in the patient, which leads to the absorption of significant amounts of radioiodine on the thyroid remnant tissue. In the absence of rhTSH levels, endogenous and exogenous TSH stimulation may be a good choice for RRA in DTC patients (46).
4.3. Pathologic Factors for Dose Determination
Dosimetric techniques are often used in challenging clinical situations, including children, the elderly, and patients with renal failure or lung cancer (15). The RRA dose is determined by the patient's characteristics and stage of the disease. This approach determines the patient's risk and the appropriate RRA therapy dose (15). The 2015 advanced technology attachment (ATA) standards include a three-tiered risk assessment method (low, intermediate, high) (5). A three-tiered risk assessment technique (low, middle, and high) is included in the 2015 ATA standards (4). A similar classification method (very low, low, high) is also recommended by the European Consensus Conference (15). Thyroid gland-confined tumors (intrathyroidal), microscopic extrathyroidal extension, macroscopic invasion into perithyroidal structures, lymph node metastasis status, number and size of metastatic lymph nodes, aggressive histology (e.g., tall cell, hobnail variant, columnar cell carcinoma), vascular invasion, and genetic mutations are all factors in the classification of thyroid gland-confined tumors (intrathyroidal) (v-raf murine sarcoma viral oncogene homolog B1 (BRAF), telomerase reverse transcriptase (TERT), etc. (5).
Due to the multiplicity of variables, the patients’ classification into three risk categories is insufficient to adequately define each patient. Within each risk category, the chance of recurrence varies according to the clinical characteristics of each patient. As a result, dosage modification may be necessary for individuals with the same risk class.
The reasons that contributed to RRA ablation failure were identical to those previously discussed.
Tamilia et al. observed, for example, that postoperative thyroid hormone withdrawal-stimulated Tg levels greater than 5 ng/mL are linked to an increased likelihood of RRA ablation failure after the administration of 1110 MBq of I-131 (47). Tg levels recorded before the administration of 3700 MBq I-131 and the ratio of this Tg level to the Tg level measured five days later were also significant predictors of RRA ablation failure, according to Bernier et al. RRA ablation has shown clinical results in Japanese patients with intermediate-to-high risk in several trials (48). Kawabe et al. reported on the success rates of RRA ablation in patients treated with 1850 MBq of I-131. In 67 patients with intermediate-to-high-risk DTC, they discovered that the first success rate was 40% based on I-131 scintigraphy data and post-RRA ablation Tg levels (2 ng/mL) in the absence of TSH stimulation (49). Watanabe et al. reported that RRA ablation with 1110 or 3700 MBq I-131 had a high success rate. They looked at 91 individuals and found that the first success rate was 15.4%, with no apparent I-131 buildup at the thyroid location and Tg levels less than 2 ng/mL after TSH stimulation (50). They calculated the success rate using I-131 scintigraphy findings and TSH-stimulated Tg levels after RRA ablation. Earlier studies utilized different RRA ablation aims than those utilized in this analysis therefore previous results cannot be compared to our results (4).
There were several limitations to this study. First and foremost, this was a retrospective and observational study with limited sample size. However, we used the inverse probability of treatment weighting (IPTW) method to reduce selection bias. Furthermore, the researchers who evaluated the clinical data were not engaged in developing the treatment procedure, reducing selection bias. Second, the low-dose and high-dose groups had distinct strategies for increasing TSH levels and the duration of the iodine insufficiency (4).
Preparation for RRA ablation, such as a low-iodine diet (47), or the use of rhTSH or thyroid hormone levels, has been demonstrated in several trials to not influence the success rate of RRA ablation (2, 4, 47-49). To determine the variations in RRA ablation success rates and the relation between RRA ablation success and clinical outcome, a long-term observational study with a high sample size should be done. Observations and clinical outcomes analysis should be kept in mind in the future (4).
Summarizing, we discovered that 70% of patients with intermediate-to-high-risk DTC successfully underwent RRA ablation. There was no discernible difference between the various recommended dosages of I-131 provided to individuals with DTC. However, high-dose treatment may be suggested in individuals with many risk factors. Tg levels before to treatment were deemed a significant risk factor for RRA ablation failure (4).
Studies comparing high- and low-dose RRA therapy for patients at low, moderate, and high risk of the disease have shown that the success rate of ablation in low-dose 1110 MBq and high-dose 3700MBq is not significantly different. This finding was also confirmed in studies by Dehbi et al. in 2019 (19), M. El-Refaei et al. in 2015 (23) Iizuka et al. in 2015 (24), and Andresen et al., in 2017 (15). In 2013, Ma et al. conducted a study with similar results (51). Furthermore, Rosario et al. (52), in 2016, studied the appropriate dosage of the drug used by patients with PTC at low risk of disease recurrence. He reported that low-dose RRA could be used to treat these patients. The study of Iizuka et al. also found no significant difference between high and low doses of RRA in high and moderate risk patients (24).
For patients with metastasis-free DTC, activity levels of 1.11 and 1.85 GBq are appropriate for residual thyroid ablation, with fewer risks of side effects than dosages of 3.7 GBq, which have dramatically different advantages. A well-designed study comparing low-activity radioactive iodine ablation to high-activity radioactive iodine ablation is required to determine the long-term adverse effects and future relapse or metastatic risk.
Some studies have suggested that the higher doses of the radioactive drug used for patients treated with RRA guarantee more success than the lower doses in patients’ treatment. For example, Campenni et al. study in Italy (34) reported that therapeutic doses of 2220 and 3700 MBq were more effective than 1110 MBq. Consistently, a 2011 study conducted by Kim et al. from Korea reported that higher doses used for RRA were associated with increased method efficiency (53). Also, Song in 2015 cited in their review that high dose RRA significantly has better outcomes compared to low dose RRA (54). A survey conducted by Bal et al. in India (2004) found that patients who received at least 25 mCi RRA were three times more likely to have successful ablation than those who received lower doses. The appropriate dose of RRA therapy for patients seems to be between 25 and 50 percent (18). Incoherence with these findings, Andresen et al. (2017) measured that appropriate ablation dosing following complete thyroidectomy is between 30 - 50 mCi (15).
In 2020, Abe et al. (35) investigated low-risk patients who used low-dose drugs in Japan and found that the patients' recovery rate was 23%, which is much lower than the success rate obtained in former studies. This can be due to the higher risk of disease in patients of Abe et al.’s study.
Another study on recurrence by Schlumberger et al. showed that recurrence did not depend on the ablation strategy. This study also supports using 1.1 GBq of radioactive iodine after receiving rhTSH for postoperative ablation in low-risk thyroid cancer patients (31). In a study by Kruijff et al. (28), he studied PTC patients and stated that high or low doses of RRA did not appear to affect disease recurrence. In a study conducted by Cai et al. in 2018, he stated that radiation thyroiditis and sialadenitis occur more at a dose of 100 mCi than 30 mCi (30). In Kim et al.’s study, the rate of side effects due to RRA was reported 2%, and Kim showed that these side effects are more common with higher dosage intake. Dysfunction of salivary glands was observed as a side effect in patients of Kim et al.’s study (17). In a similar study conducted by Patel in 2013 to evaluate the side effects of high-dose RRA, various side effects were reported, including the possibility of secondary malignancy and impaired fertility in patients. To use high doses, it is better to weigh the advantages of the method against the disadvantages of using it so that with the least side effects, at the same time, side effects such as recurrence do not occur after treatment (29).
In a review article by Cherk et al. study (32) in 2008, it has been acknowledged that due to the additional side effects of a high dose RRA, 3700 MBq, a low dose of 1110 MBq is preferred.
Regarding disease-free survival (DFS) in patients, Kim et al. performed ablation with different doses of 30, 80, and 150 mCi in categorized three groups of group A, group B, and group C, respectively. He discovered that DFS has significantly improved in the higher dose group, 81.7% of cases in group A, 89.5% of cases in group B, and 94.8% of cases in group C (P < 0.001). In 9.8% of cases, there was an average clinical recurrence during 6.6 years of follow-up (17). However, DFS was not associated with patient dosing. The researcher found that the patient's dosage should be determined based on the risk of recurrence in patients, thus minimizing the exposure to unnecessary radiation and maximizing the effectiveness of treatment (17). In another study performed by Samuel and Rajashekharrao in 1994 to determine the appropriate dosage for RRA, he recommended that the initial dose and initial tissue mass should be determined for successful treatment response (22).
In a study conducted in 2018 by Abuqbeitah et al. (36), he stated that the maximum dose administered empirically to a patient is 250 mCi when it is not possible to determine the exact RRA dosage required for the patient.
4.4. Conclusion
Radioiodine therapy (RRA) has been used as a thyroid tissue remnant ablation to remove thyroid tissue for about 70 years and showed a high therapeutic efficacy. Usually, an empirical high-dose RRA is prescribed to treat patients. In this review, we investigated 21 studies and found that treatment efficacy of low and high-dose therapy do not differ significantly in low-to-moderate risk patients; however, the use of high-dose RRA produces a better and statistically significant response in high-risk patients. We recommend the clinicians carefully weigh the benefits and harms of RRA prior to prescribing a high-dose therapy since it may cause serious complications, including a higher risk of secondary malignancy and fertility incompetence.
4.5. Limitation
Studies have not comprehensively addressed other side effects of RRA such as cardiovascular complications.
4.6. Recommendation
It is recommended that scientists conduct studies with long-term follow-up to ascertain the side effects of radiopharmaceuticals.