1. Background
Nowadays, cancer is one of the leading causes of death worldwide, and discovering new anticancer agents with high selectivity and low adverse effects is a significant challenge encountered by researchers (1, 2). Receptor protein tyrosine kinases (RPTKs), also called phosphotyrosine kinases, have a considerable role in the signal transduction pathway and are responsible for regulating various functions in normal cells, including cell growth, proliferation, metabolism, differentiation, and apoptosis (3, 4). The alteration of this enzyme via mutations would lead to dysregulated cell cycle and uncontrolled cell duplication, defined as cancer (5-7). The FDA approved 47 kinase inhibitors over the last 30 years that have developed as anticancer agents (8).
The human epidermal growth factor receptors (EGFRs) belong to a class of RPTKs, including four members: EGFR/ERBB1/HER1, ERBB2/HER2, ERBB3/HER3 and ERBB4/HER4 (9). This type of receptors can perform a key role in signal transduction for adjusting cell-growth and developing many types of solid tumors, including head and neck, lung, breast, bladder, prostate, and kidney cancers (10-12).
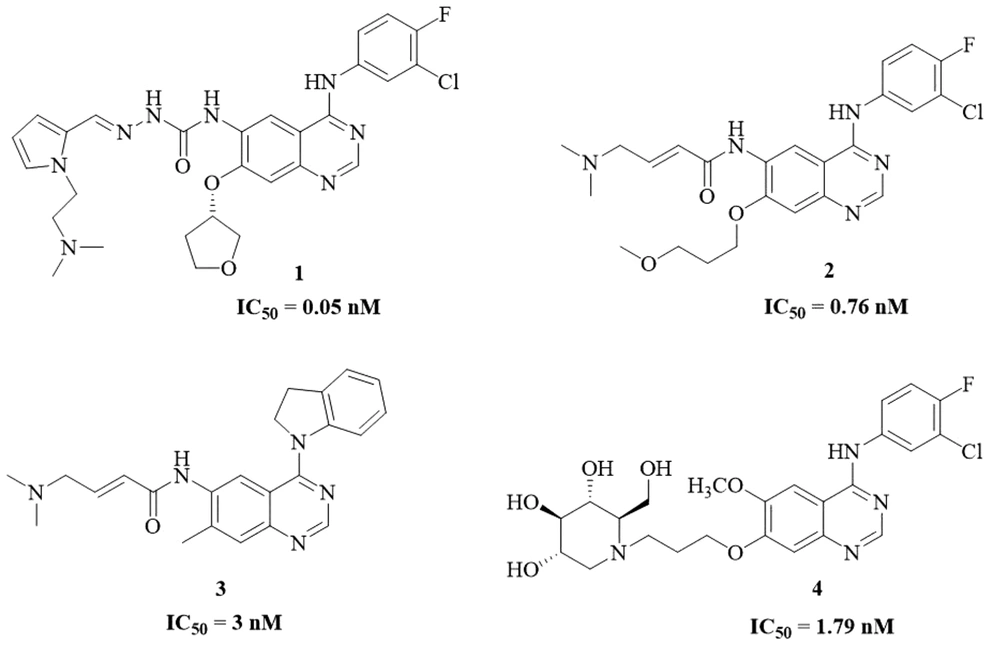
As a relatively lethal disease, lung cancer’s manifestations are categorized into two main groups: small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC) (13, 14). This invasive and metastatic cancer type and stage would guide the specialist to select proper treatment, including surgery, radiation, and chemotherapy. Erlotinib (Tarceva®), an EGFR tyrosine kinase inhibitor (EGFR-TKI), has been approved as the first-line therapy for EGFR mutated progressive or metastatic NSCLC (15). Quinazolines are ubiquitous heterocyclic scaffolds found in various pharmaceuticals and natural products (16). The quinazoline-containing derivatives have been contributed to the variety of EGFR-TKIs. There have been many novel quinazoline derivatives possessing high inhibitory activity compared to FDA approved drugs in the last few years. Some novel and potent EGFR-TKIs with quinazoline moiety were illustrated in Figure 1. Compound 1 is a semicarbazone fused quinazoline group derivative with an IC50 value of 0.05 nM (17). The concept behind the designing compound 2 (IC50 = 0.76 nM) was changing the size of alkoxyalkane chains at 7-position of quinazoline core (18). Compound 3 (IC50 = 3 nM) bearing 2,3-dihydro-indolequinoline core demonstrated strong potency as well as excellent antiproliferative activity (19). Compound 4 (IC50 = 1.79 nM) was designed as dual EGFR and α-glucosidase inhibitor possessing quinazoline moiety hybridized with 1-deoxynojirimycin segment (20).
Based on studies, drug resistance in cancer and ineffective chemotherapy mainly stem from EGFR mutation (replacement of the threonine 790 with methionine 790), leading to the progression of cancer stages. Although this factor could be conquered through developing inhibitors binding covalently to the ATP binding site of EGFR (21), these inhibitors suffer from weak selectivity due to their affinity to other kinases (22). The alternative strategy introduces reversible EGFR-TKIs, which have been desirable recently, and researchers are working on them.
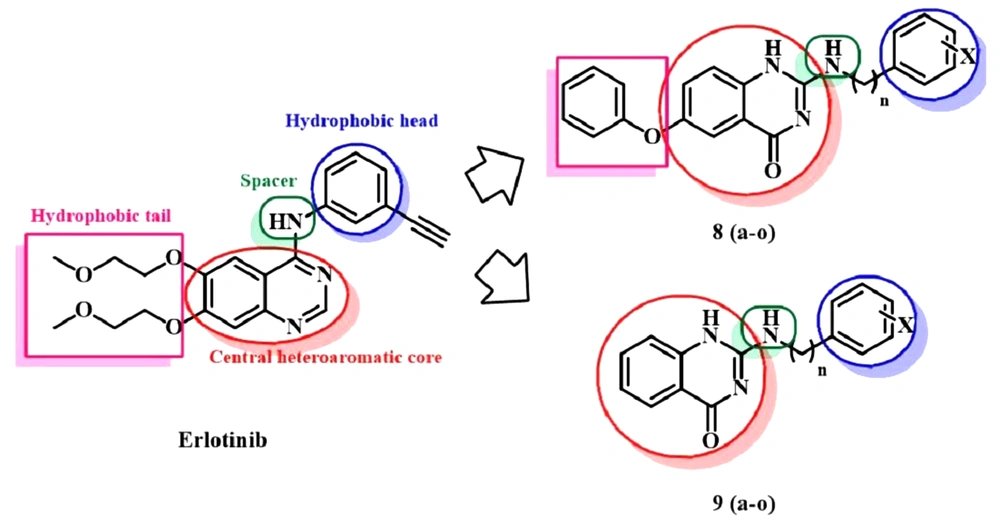
Considering the necessary features of erlotinib as a reversible inhibitor and related structures, we designed two series of novel quinazolinone derivatives as EGFR-TKIs illustrated in Figure 2. According to the essential pharmacophoric features of erlotinib (3), a hydrophobic head and tail on a heteroaromatic system and a hydrogen acceptor spacer that bridges between head and heteroaromatic system are required to locate a compound into the active site of EGFR tyrosine kinase (EGFR-TK). Therefore, the modifications of our novel series of quinazolinone derivatives (compounds 8 and 9) included the introduction of various N-aryl and -alkyl groups (hydrophobic head) with different substituents at the 2-position of quinazolinone moiety (bioisoster of quinazoline group) and the amino group served as a spacer. Besides, hydrogen at the 6-position was replaced with phenoxy group in 8a-o derivatives to resemble a hydrophobic tail (Figure 2). Finally, the activities of the novel compounds toward recombinant EGFR-TK were evaluated.
2. Methods
2.1. Chemistry
All reagents and solvents were purchased from Aldrich or Merck Company and utilized without any purification. IR spectra were measured on a Perkin Elmer 834 spectrometer. The absorptions were reported on the wavenumber (cm-1) scale, in the range 400 - 4000 cm-1. All the 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance II spectrophotometer using CDCl3 as a solvent and tetramethylsilane as an internal standard. Finnigan-MAT-8430EI-MS was applied to obtain mass spectra; at 70 eV; in m/z (rel.%). Melting points were obtained using Electrothermal 9100 apparatus and were uncorrected.
2.2. General Procedure for the Synthesis of Compounds 8a-o
A mixture of 2-amino-5-fluorobenzoic acid (5.0 mmol), phenol (5.2 mmol), CuI (0.1 mmol), NaH (2.0 mmol) in DMF (3 mL) was refluxed at 110°C for 2 h. After the addition of ethyl chloroformate (6.0 mmol) to the medium, Et3N (2.0 mmol) was added dropwise over 10 min at room temperature and refluxed at 110°C for 3 h. Then, the mixture was cooled, diluted with CH2Cl2 (2 mL) and aqueous NH4Cl solution (3 mL), and stirred for 30 min. The aqueous layer was extracted with CH2Cl2, dried over sodium sulfate anhydrous, and concentrated under reduced pressure. The precipitate was recrystallized from EtOH (30 mL) to afford 6-phenoxyisatoic anhydride.
To a mixture of appropriate aniline or benzylamine (3.0 mmol) and trichloroacetonitrile (3.5 mmol), a mixture of synthesized 6-phenoxyisatoic anhydride (3.5 mmol) and CuO (0.1 mmol) in DMF (3 mL) was slowly added and refluxed at 110°C for 4 h. The end of the reaction was controlled by TLC (eluent: AcOEt/ hexane 1:3). Then, the mixture was diluted with CH2Cl2 (2 mL) and aqueous NH4Cl solution (3 mL) and stirred for 30 min. The aqueous layer was extracted with CH2Cl2 dried over sodium sulfate anhydrous and concentrated under reduced pressure. The precipitate was washed with diethyl ether to afford final product 8.
2.2.1. 2-(Benzylamino)-6-phenoxyquinazolin-4(1H)-one (8a)
White powder; yield: 0.30 g (88%); mp: 199 - 201°C. IR (KBr) (νmax, cm-1): 3201, 3101, 1651, 1644, 1124, 1100. 1H-NMR (500MHz, CDCl3): δH = 4.02 (2 H, s, CH2), 6.08 (1 H, s, NH), 7.29 - 7.36 (7 H, m, H3,4,5-phenoxy, H5-quinazolinone, H3,4,5-benzylamine), 7.40 (1 H, d, 3J = 7.9 Hz, H7-quinazolinone), 7.51 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.78 (2 H, d, 3J = 7.5 Hz, H2,6-phenoxy), 7.93 (2 H, d, 3J = 7.8 Hz, H2,6-benzylamine), 9.97 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 45.9 (CH2), 127.4 (2 CH), 128.7 (2 CH), 129.3 (CH), 130.1 (2 CH), 130.7 (2 CH), 132.5 (CH), 132.9 (CH), 133.0 (2 CH), 134.6 (C), 135.7 (C), 142.1 (C), 146.0 (C), 147.3 (C), 166.2 (C), 176.0 (C). EI-MS: 343 (M+, 14), 266 (58), 250 (20), 238 (77), 211 (59), 133 (100), 106 (44), 93 (55).
2.2.2. 2-((2-Chlorobenzyl)amino)-6-phenoxyquinazolin-4(1H)-one (8b)
White powder; yield: 0.34 g (89%); mp: 234 - 236°C. IR (KBr) (νmax, cm-1): 3458, 3324, 1658, 1625, 1254, 1010. 1H-NMR (500MHz, CDCl3): δH = 4.45 (2 H, s, CH2), 6.01 (1 H, s, NH), 7.36 (1 H, t, 3J = 7.9 Hz, H4-phenoxy), 7.47 - 7.51 (7 H, m, H2,3,5,6-phenoxy, H5,7-quinazolinone, H5-benzylamine), 7.60 (1 H, t, 3J = 7.9 Hz, H4-benzylamine), 7.91 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.97 (2 H, d, 3J = 7.5 Hz, H3,6- benzylamine), 9.99 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 46.6 (CH2), 127.5 (CH), 128.8 (2 CH), 129.0 (CH), 129.2 (CH), 129.3 (CH), 130.7 (CH), 131.6 (2 CH), 131.8 (2 CH), 131.9 (CH), 132.0 (C), 134.4 (C), 134.5 (C), 140.3 (C), 142.1 (C), 147.2 (C), 161.2 (C), 172.7 (C). EI-MS: 377 (M+, 3), 266 (19), 252 (35), 238 (29), 211 (60), 168 (55), 111 (100), 93 (52).
2.2.3. 2-((4-Methoxybenzyl)amino)-6-phenoxyquinazolin-4(1H)-one (8c)
White powder; yield: 0.30 g (81%); mp: 245 - 247°C. IR (KBr) (νmax, cm-1): 3421, 3320, 1651, 1620, 1204, 1000. 1H-NMR (500MHz, CDCl3): δH = 3.36 (3 H, s, OCH3), 4.45 (2 H, s, CH2), 6.11 (1 H, s, NH), 7.28 - 7.36 (5 H, m, H2,6-phenoxy, H7-quinazolinone, H3,5-benzylamine), 7.47-7.50 (4 H, m, H3,5-phenoxy, H5,8-quinazolinone), 7.61 (1 H, t, 3J = 7.9 Hz, H4-phenoxy), 7.96 (2 H, d, 3J = 7.8 Hz, H2,6-benzylamine), 9.00 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 47.5 (CH2), 58.2 (OCH3), 128.8 (CH), 129.2 (2 CH), 129.4 (2 CH), 132.5 (2 CH), 133.2 (CH), 133.3 (CH), 134.1 (C), 136.1 (2 CH), 137.6 (CH), 138.1 (C), 139.7 (C), 140.0 (C), 145.2 (C), 150.3 (C), 161.2 (C), 173.3 (C). EI-MS: 373 (M+, 12), 266 (24), 252 (44), 136 (39), 122 (44), 107 (100), 93 (55).
2.2.4. 2-((4-Chlorobenzyl)amino)-6-phenoxyquinazolin-4(1H)-one (8d)
Gray powder; yield: 0.31g (83%); mp: 251 - 253°C. IR (KBr) (νmax, cm-1): 3524, 3399, 1693, 1679, 1254, 1025. 1H-NMR (500MHz, CDCl3): δH = 4.06 (2 H, s, CH2), 6.13 (1 H, s, NH), 7.26 - 7.31 (5 H, m, H2,6-phenoxy, H3,5-benzylamine, H5-quinazolinone), 7.44 (2 H, d, 3J = 7.9 Hz, H7,8-quinazolinone), 7.57 (1 H, t, 3J = 7.9 Hz, H4-phenoxy), 7.69 (2 H, t, 3J = 7.9 Hz, H3,5-phenoxy), 7.87 (2 H, d, 3J = 7.8 Hz, H2,6-benzylamine), 8.06 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 47.6 (CH2), 123.5 (2 CH), 127.4 (CH), 128.8 (CH), 129.3 (2 CH), 130.2 (2 CH), 132.5 (CH), 133.1 (C), 135.7 (2 CH), 136.6 (CH), 137.0 (C), 139.9 (C), 144.7 (C), 146.2 (C), 151.3 (C), 161.3 (C), 167.0 (C). EI-MS: 379 (M+2, 6), 377 (12), 284 (24), 266 (29), 252 (40), 111 (100), 93 (70).
2.2.5. 2-((4-Fluorobenzyl)amino)-6-phenoxyquinazolin-4(1H)-one (8e)
White powder; yield: 0.28 g (77%); mp: 220 - 222°C. IR (KBr) (νmax, cm-1): 3449, 3306, 1690, 1672, 1258, 1024. 1H-NMR (500MHz, CDCl3): δH = 4.13 (2 H, s, CH2), 6.23 (1 H, s, NH), 7.29 - 7.33 (6 H, m, H2,4,6-phenoxy, H5-quinazolinone, H3,5-benzylamine), 7.40 (1 H, d, 3J = 7.9 Hz, H7-quinazolinone), 7.48 - 7.51 (4 H, m, H2,6-benzylamine, H3,5-phenoxy), 7.92 (1 H, d, 3J = 7.8 Hz, H8-quinazolinone), 8.73 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 47.2 (CH2), 122.5 (2 CH), 127.5 (2 CH), 128.7 (CH), 129.2 (CH), 130.7 (2 CH), 132.5 (CH), 133.0 (2 CH), 134.7 (CH), 135.6 (C), 136.6 (C), 137.7 (C), 142.1 (C), 144.5 (C), 147.3 (C), 161.2 (C), 169.0 (C). EI-MS: 361 (M+, 9), 266 (15), 252 (24), 238 (25), 124 (36), 110 (100), 95 (85), 93 (41).
2.2.6. 2-((4-Methylbenzyl)amino)-6-phenoxyquinazolin-4(1H)-one (8f)
White powder; yield: 0.26 g (72%); mp: 229 - 231°C. IR (KBr) (νmax, cm-1): 3455, 3325, 1688, 1654, 1287, 1021. 1H-NMR (500MHz, CDCl3): δH = 2.66 (3 H, s, CH3), 4.46 (2 H, s, CH2), 6.19 (1 H, s, NH), 7.21 (1 H, d, 3J = 7.8 Hz, H7-quinazolinone), 7.22 - 7.26 (6 H, m, H3,4,5-phenoxy, H5-quinazolinone, H3,5-benzylamine), 7.33 (2 H, d, 3J = 7.5 Hz, H2,6-phenoxy), 7.35 (1 H, d, 3J = 7.8 Hz, H8-quinazolinone), 7.91 (2 H, d, 3J = 7.5 Hz, H2,6-benzylamine), 9.02 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 22.2 (CH3), 45.7 (CH2), 126.2 (C), 127.5 (2 CH), 127.7 (CH), 130.0 (CH), 130.6 (CH), 131.3 (2 CH), 132.2 (2 CH), 133.0 (CH), 134.0 (2 CH), 134.9 (CH), 137.6 (C), 138.8 (C), 140.6 (C), 143.4 (C), 161.3 (C), 171.1 (C). EI-MS: 357 (M+, 15), 266 (19), 252 (21), 238 (100), 120 (54), 93 (88).
2.2.7. 6-Phenoxy-2-(phenylamino)quinazolin-4(1H)-one (8g)
White powder; yield: 0.24 g (74%); mp: 214 - 216°C. IR (KBr) (νmax, cm-1): 3465, 3385, 1681, 1657, 1285, 1064. 1H-NMR (500MHz, CDCl3): δH = 6.22 (1 H, s, NH), 7.30 - 7.36 (8 H, m, H3,4,5-phenyl, H3,4,5-phenoxy, H5,7-quinazolinone), 7.40 (1 H, d, 3J = 7.8 Hz, H8-quinazolinone), 7.50 (2 H, d, 3J = 7.5 Hz, H2,6-phenoxy), 7.93 (2 H, d, 3J = 7.9 Hz, H2,6-phenyl), 8.12 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.7 (2 CH), 127.3 (2 CH), 127.8 (CH), 128.7 (CH), 129.7 (2 CH), 130.7 (2 CH), 132.5 (CH), 133.0 (CH), 134.0 (C), 134.9 (CH), 136.7 (C), 140.1 (C), 142.4 (C), 148.4 (C), 160.7 (C), 171.2 (C). EI-MS: 329 (M+, 14), 238 (12), 236 (19), 93 (78), 92 (100), 77 (52).
2.2.8. 2-((2-Nitrophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8h)
Cream powder; yield: 0.31 g (82%); mp: 258 - 260°C. IR (KBr) (νmax, cm-1): 3566, 3306, 1696, 1665, 1556, 1357, 1235, 1016. 1H-NMR (500MHz, CDCl3): δH = 6.04 (1 H, s, NH), 7.26 - 7.33 (7 H, m, H3,4,5-phenoxy, H4,5,6-phenyl, H5-quinazolinone), 7.43 (2 H, d, 3J = 7.8 Hz, H2,6-phenoxy), 7.47 (2 H, d, 3J = 7.8 Hz, H7,8-quinazolinone), 7.89 (1 H, d, 3J = 7.5 Hz, H3-phenyl), 8.32 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 128.9 (CH), 129.3 (2 CH), 129.7 (CH), 130.8 (2 CH), 132.8 (2 CH), 133.0 (CH), 133.2 (CH), 133.5 (C), 134.1 (C), 134.5 (2 CH), 138.7 (C), 140.5 (C), 142.0 (C), 148.3 (C), 160.6 (C), 170.2 (C). EI-MS: 374 (M+, 12), 281 (19), 252 (59), 137 (57), 123 (100), 93 (71).
2.2.9. 2-((4-Nitrophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8i)
Green powder; yield: 0.33 g (89%); mp: 273 - 275°C. IR (KBr) (νmax, cm-1): 3425, 3325, 1688, 1654, 1553, 1324, 1236, 1000. 1H-NMR (500MHz, CDCl3): δH = 6.17 (1 H, s, NH), 7.29 - 7.35 (5 H, m, H2,4,6-phenoxy, H5,7-quinazolinone), 7.48 (2 H, d, 3J = 7.8 Hz, H2,6-phenyl), 7.82 - 7.85 (4 H, m, H3,5-phenyl, H3,5-phenoxy), 7.91 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 9.10 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 127.4 (2 CH), 128.7 (2 CH), 129.2 (2 CH), 130.1 (CH), 132.6 (CH), 135.7 (2 CH), 136.2 (CH), 136.9 (C), 137.1 (CH), 138.0 (C), 138.7 (C), 140.3 (C), 144.7 (C), 149.4 (C), 162.7 (C), 171.6 (C). EI-MS: 374 (M+, 15), 281 (25), 252 (52), 137 (54), 123 (100), 93 (66).
2.2.10. 2-((3-Nitrophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8j)
Pale yellow powder; yield: 0.32 g (86%); mp: 263-265 °C. IR (KBr) (νmax, cm-1): 3583, 3254, 1688, 1627, 1568, 1377, 1220, 1033. 1H-NMR (500MHz, CDCl3): δH = 6.20 (1 H, s, NH), 7.29 - 7.34 (4 H, m, H2,4,6-phenoxy, H7-quinazolinone), 7.47 (1 H, d, 3J = 7.8 Hz, H6-phenyl), 7.60 - 7.63 (5 H, m, H4-phenyl, H3,5-phenoxy, H5,8-quinazolinone), 7.74 (1 H, t, 3J = 7.9 Hz, H5-phenyl), 8.04 (1 H, s, H2-phenyl), 9.12 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 127.6 (CH), 128.8 (CH), 129.3 (CH), 130.2 (2 CH),130.3 (2 CH), 132.7 (CH), 135.8 (2 CH), 136.2 (C), 136.9 (CH), 137.1 (CH), 138.2 (C), 139.9 (C), 141.1 (C), 145.6 (C), 150.0 (C), 161.7 (C), 170.2 (C). EI-MS: 374 (M+, 10), 281 (21), 252 (50), 137 (65), 123 (100), 93 (81).
2.2.11. 2-((4-Bromophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8k)
Cream powder; yield: 0.33 g (82%); mp: 244 - 246°C. IR (KBr) (νmax, cm-1): 3578, 3365, 1658, 1623, 1258, 1026. 1H-NMR (500MHz, CDCl3): δH = 6.10 (1 H, s, NH), 6.92 (1 H, t, 3J = 7.8 Hz, H4-phenoxy), 7.03 (2 H, t, 3J = 7.9 Hz, H3,5-phenoxy), 7.14 (2 H, d, 3J = 7.9 Hz, H2,6-phenyl), 7.24 (1 H, d, 3J = 7.9 Hz, H7-quinazolinone), 7.30 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.56 - 7.60 (5 H, m, H3,5-phenyl, H2,6-phenoxy, H5-quinazolinone), 8.65 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.8 (2 CH), 127.8 (CH), 129.7 (CH), 130.1 (2 CH), 133.8 (CH), 135.2 (CH), 138.0 (4 CH), 138.2 (C), 138.6 (C), 139.7 (C), 140.3 (C), 145.7 (C), 155.1 (C), 162.3 (C), 171.2 (C). EI-MS: 407 (M+, 20), 252 (22), 238 (34), 169 (62), 155 (100), 93 (29).
2.2.12. 2-((3,4-Dichlorophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8l)
White powder; yield: 0.36 g (91%); mp: 275 - 277°C. IR (KBr) (νmax, cm-1): 3548, 3389, 1688, 1654, 1237, 1110. 1H-NMR (500MHz, CDCl3): δH = 6.00 (1 H, s, NH), 7.02 (1 H, t, 3J = 7.6 Hz, H4-phenoxy), 7.05 - 7.08 (4 H, m, H2,6-phenoxy, H6-phenyl, H7-quinazolinone), 7.38 - 7.43 (4 H, m, H3,5-phenoxy, H2-phenyl, H5-quinazolinone), 7.76 (1 H, d, 3J = 7.9 Hz, H5-phenyl), 7.91 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 8.65 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 127.9 (CH), 128.2 (CH), 129.3 (2 CH), 129.7 (2 CH), 129.8 (CH), 130.1 (C), 130.7 (CH), 138.2 (CH), 138.6 (2 CH), 139.9 (C), 140.1 (C), 145.2 (C), 147.2 (C), 149.0 (C), 155.2 (C), 165.3 (C), 174.4 (C). EI-MS: 397 (M+, 2), 304 (24), 252 (32), 159 (60), 145 (100), 93 (24).
2.2.13. 2-((4-Fluorophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8m)
Cream powder; yield: 0.27 g (78%); mp: 219 - 221°C. IR (KBr) (νmax, cm-1): 3355, 3325, 1687, 1625, 1254, 1013. 1H-NMR (500MHz, CDCl3): δH = 6.23 (1 H, s, NH), 7.29 (1 H, t, 3J = 7.8 Hz, H4-phenoxy), 7.30 - 7.36 (7 H, m, H3,5-phenoxy, H2,3,5,6-phenyl, H5-quinazolinone), 7.40 (1 H, d, 3J = 7.9 Hz, H7-quinazolinone), 7.51 (2 H, d, 3J = 7.9 Hz, H2,6- phenoxy), 7.92 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 8.65 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.2 (2 CH), 127.5 (2 CH), 127.7 (CH), 128.7 (2 CH), 129.2 (CH), 130.0 (CH), 130.7 (CH), 132.6 (C), 138.6 (2 CH), 138.9 (C), 139.4 (C), 142.2 (C), 147.2 (C), 150.1 (C), 160.3 (C), 172.2 (C). EI-MS: 347 (M+, 3), 254 (25), 238 (54), 110 (62), 96 (100), 93 (45).
2.2.14. 2-((4-Chlorophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8n)
Cream powder; yield: 0.31 g (86%); mp: 248 - 250°C. IR (KBr) (νmax, cm-1): 3524, 3325, 1685, 1675, 1245, 1019. 1H-NMR (500MHz, CDCl3): δH = 6.13 (1 H, s, NH), 6.88 (1 H, t, 3J = 7.8 Hz, H4-phenoxy), 7.11 (2 H, d, 3J = 7.9 Hz, H2,6-phenoxy), 7.23 - 7.26 (4 H, m, H2,3,5,6-phenyl), 7.28 - 7.35 (3 H, m, H3,5-phenoxy, H5-quinazolinone), 7.56 (2 H, d, 3J = 7.9 Hz, H7,8-quinazolinone), 8.65 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.6 (2 CH), 129.3 (CH), 129.7 (CH), 134.0 (2 CH), 137.4 (2 CH), 138.4 (CH), 138.5 (C), 138.6 (CH), 139.2 (2 CH), 139.5 (C), 139.8 (C), 143.4 (C), 147.8 (C), 151.1 (C), 160.3 (C), 173.3 (C). EI-MS: 365 (M+2, 7), 363 (13), 252 (29), 238 (41), 126 (62), 112 (100), 93 (44).
2.2.15. 2-((3-Chlorophenyl)amino)-6-phenoxyquinazolin-4(1H)-one (8o)
White powder; yield: 0.31 g (84%); mp: 237 - 239°C. IR (KBr) (νmax, cm-1): 3578, 3325, 1688, 1674, 1299, 1124. 1H-NMR (500MHz, CDCl3): δH = 6.02 (1 H, s, NH), 6.86 (1 H, t, 3J = 7.8 Hz, H5-phenyl), 7.03 - 7.06 (7 H, m, H2,6-phenyl, H5-quinazolinone, H2,3,5,6-phenoxy), 7.12 (1 H, d, 3J = 7.9 Hz, H4-phenyl), 7.19 (1 H, t, 3J = 7.9 Hz, H4-phenoxy), 7.27 (1 H, d, 3J = 7.9 Hz, H7-quinazolinone), 7.56 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 8.52 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.5 (2 CH), 129.7 (CH), 129.9 (2 CH), 130.1 (2 CH), 132.6 (C), 135.2 (CH), 136.0 (CH), 137.0 (CH), 138.1 (2 CH), 138.3 (C), 138.9 (C), 143.4 (C), 145.2 (C), 148.1 (C), 162.5 (C), 172.5 (C). EI-MS: 365 (M+2, 9), 363 (18), 252 (35), 238 (49), 126 (65), 112 (100), 93 (77).
2.3. General Procedure for the Synthesis of Compounds 9a-o
A mixture of 2-amino-benzoic acid (5.0 mmol) and ethyl chloroformate (6.0 mmol) in DMF (3 ml) was refluxed at 110°C for 3 h. Et3N (2.0 mmol) was added to the reaction dropwise over 10 min at room temperature and refluxed at 110 °C for 3 h. Then, the mixture was cooled, diluted with CH2Cl2 (2 mL) and aqueous NH4Cl solution (3 mL), and stirred for 30 min. The aqueous layer was extracted with CH2Cl2, dried over sodium sulfate anhydrous, and concentrated under reduced pressure. The precipitate was recrystallized from EtOH (30 mL) to afford isatoic anhydride.
To a mixture of appropriate aniline or benzylamine (3.0 mmol) and trichloroacetonitrile (3.5 mmol), a mixture of synthesized isatoic anhydride (3.5 mmol) and CuO (0.1 mmol) in DMF (3 mL) was slowly added and refluxed at 110°C for 4 h. TLC (eluent: AcOEt/ hexane 1:3) was applied to determine the end of the reaction. The mixture was diluted with CH2Cl2 (2 mL) and aqueous NH4Cl solution (3 mL) and stirred for 30 min. The aqueous layer was extracted with CH2Cl2 dried over sodium sulfate anhydrous and concentrated under reduced pressure. The precipitate was washed with diethyl ether to afford final product 9.
2.3.1. 2-(Benzylamino)quinazolin-4(1H)-one (9a)
White powder; yield: 0.21 g (82%); mp: 158 - 160°C. IR (KBr) (νmax, cm-1): 3443, 3301, 1691, 1677, 1014. 1H-NMR (500MHz, CDCl3): δH = 4.13 (2 H, s, CH2), 6.08 (1 H, s, NH), 7.26 - 7.30 (3 H, m, H6-quinazolinone, H3,5-benzylamine), 7.44 (2 H, d, 3J = 7.4 Hz, H5,8-quinazolinone), 7.59 (1 H, t, 3J = 7.9 Hz, H7-quinazolinone), 7.67 (1 H, t, 3J = 7.9 Hz, H4-benzylamine), 7.87 (2 H, d, 3J = 7.8 Hz, H2,6-benzylamine), 8.06 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 47.6 (CH2), 123.5 (2 CH), 127.0 (CH), 127.4 (CH), 128.8 (CH), 130.2 (2 CH), 132.5 (CH), 133.1 (C), 135.7 (CH), 144.7 (C), 146.0 (C), 161.3 (C), 167.0 (C). EI-MS: 251 (M+, 12), 146 (33), 133 (29), 119 (78), 106 (100), 77 (25).
2.3.2. 2-((2-Chlorobenzyl)amino)quinazolin-4(1H)-one (9b)
Gray powder; yield: 0.24 g (84%); mp: 169 - 171°C. IR (KBr) (νmax, cm-1): 3404, 3324, 1688, 1671, 1010. 1H-NMR (500MHz, CDCl3): δH = 4.12 (2 H, s, CH2), 6.21 (1 H, s, NH), 7.29 - 7.35 (5 H, m, H4,5,6-benzylamine, H6,7-quinazolinone), 7.38 (1 H, d, 3J = 7.8 Hz, H8-quinazolinone), 7.50 (1 H, d, 3J = 7.9 Hz, H5-quinazolinone), 7.91 (1 H, d, 3J = 7.9 Hz, H3-benzylamine), 8.77 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 47.2 (CH2), 125.6 (3 CH), 127.5 (2 CH), 128.8 (CH), 129.2 (CH), 130.7 (CH), 132.5 (C), 142.1 (C), 144.5 (C), 147.3 (C), 161.2 (C), 169.7 (C). EI-MS: 287 (M+2, 4), 285 (8), 174 (19), 167 (21), 146 (22), 126 (62), 119 (75), 111 (100).
2.3.3. 2-((4-Methoxybenzyl)amino)quinazolin-4(1H)-one (9c)
Cream powder; yield: 0.22 g (80%); mp: 149 - 151°C. IR (KBr) (νmax, cm-1): 3368, 3299, 1690, 1663, 1011. 1H-NMR (500MHz, CDCl3): δH = 3.55 (3 H, s, OCH3), 4.20 (2 H, s, CH2), 6.12 (1 H, s, NH), 7.30 - 7.34 (4 H, m, H3,5-benzylamine, H6,7-quinazolinone), 7.41 (2 H, d, 3J = 7.8 Hz, H2,6-benzylamine), 7.50 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.92 (1 H, d, 3J = 7.8 Hz, H5-quinazolinone), 9.00 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 47.7 (CH2), 58.6 (OCH3), 126.7 (2 CH), 127.0 (CH), 127.3 (CH), 128.7 (2 CH), 129.7 (CH), 130.7 (CH), 132.5 (C), 136.7 (C), 142.4 (C), 145.3 (C), 160.7 (C), 170.0 (C). EI-MS: 281 (M+, 3), 174 (24), 167 (44), 146 (39), 119 (100), 107 (35).
2.3.4. 2-((4-Chlorobenzyl)amino)quinazolin-4(1H)-one (9d)
White powder; yield: 0.25 g (88%); mp: 174 - 176°C. IR (KBr) (νmax, cm-1): 3452, 3300, 1688, 1672, 1000. 1H-NMR (500MHz, CDCl3): δH = 4.15 (2 H, s, CH2), 6.00 (1 H, s, NH), 7.28 - 7.33 (4H, m, H2,6-benzylamine, H6,7-quinazolinone), 7.44 (1H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.47 (1 H, d, 3J = 8.0 Hz, H5-quinazolinone), 7.89 (2 H, d, 3J = 7.9 Hz, H3,5-benzylamine), 8.57 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 46.2 (CH2), 128.9 (2 CH), 129.3 (CH), 129.7 (2 CH), 130.8 (2 CH), 132.8 (CH), 132.9 (C), 135.7 (C), 141.9 (C), 143.6 (C), 160.6 (C), 170.1 (C). EI-MS: 287 (M+2, 4), 285 (9), 174 (20), 167 (29), 146 (42), 119 (55), 111 (100).
2.3.5. 2-((4-Fluorobenzyl)amino)quinazolin-4(1H)-one (9e)
White powder; yield: 0.23 g (87%); mp: 151 - 153°C. IR (KBr) (νmax, cm-1): 3463, 3365, 1688, 1623, 1025. 1H-NMR (500MHz, CDCl3): δH = 4.05 (2 H, s, CH2), 5.89 (1 H, s, NH), 7.38 (2 H, d, 3J = 7.9 Hz, H3,5-benzylamine), 7.42 (1 H, d, 3J = 8.0 Hz, H8-quinazolinone), 7.52 - 7.60 (2 H, m, H6,7-quinazolinone), 7.80 (1 H, d, 3J = 8.0 Hz, H5-quinazolinone), 7.93 (2 H, d, 3J = 7.9 Hz, H2,6-benzylamine), 8.35 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 42.9 (CH2), 127.9 (CH), 128.2 (CH), 129.5 (CH), 130.0 (CH), 131.4 (2 CH), 133.3 (2 CH), 135.9 (C), 142.8 (C), 144.2 (C), 148.0 (C), 164.6 (C), 174.7 (C). EI-MS: 269 (M+, 9), 174 (15), 151 (20), 146 (31), 124 (36), 110 (100), 95 (85).
2.3.6. 2-((4-Methylbenzyl)amino)quinazolin-4(1H)-one (9f)
White powder; yield: 0.21 g (79%); mp: 148 - 150°C. IR (KBr) (νmax, cm-1): 3425, 3256, 1658, 1626, 1045. 1H-NMR (500MHz, CDCl3): δH = 2.67 (3 H, s, CH3), 4.46 (2 H, s, CH2), 6.19 (1 H, s, NH), 7.21 (1 H, d, 3J = 7.6 Hz, H8-quinazolinone), 7.23 (1 H, t, 3J = 7.6 Hz, H6-quinazolinone), 7.28 (2 H, d, 3J = 7.6 Hz, H3,5-benzylamine), 7.34 (1 H, d, 3J = 7.6 Hz, H5-quinazolinone), 7.41 (1 H, t, 3J = 7.6 Hz, H7-quinazolinone), 7.92 (2 H, d, 3J = 7.6 Hz, H2,6-benzylamine), 9.06 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 22.2 (CH3), 45.7 (CH2), 125.1 (C), 126.2 (2 CH), 127.5 (CH), 127.7 (2 CH), 129.1 (C), 130.0 (CH), 130.6 (CH), 138.8 (CH), 140.6 (C), 143.4 (C), 160.0 (C), 171.1 (C). EI-MS: 265 (M+, 10), 174 (12), 147 (42), 120 (100), 119 (33), 91 (88).
2.3.7. 2-(Phenylamino)quinazolin-4(1H)-one (9g)
Cream powder; yield: 0.19 g (80%); mp: 144-146°C. IR (KBr) (νmax, cm-1): 3410, 3321, 1641, 1620, 1055. 1H-NMR (500MHz, CDCl3): δH = 6.12 (1 H, s, NH), 7.29 - 7.35 (3 H, m, H6,8-quinazolinone, H4-phenyl), 7.48 (2 H, d, 3J = 7.4 Hz, H2,6-phenyl), 7.82-7.85 (3 H, m, H3,5-phenyl, H7-quinazolinone,), 7.91 (1 H, d, 3J = 7.9 Hz, H5-quinazolinone), 9.22 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 127.4 (2 CH), 127.9 (2 CH), 128.7 (2 CH), 129.2 (CH), 130.1 (CH), 132.6 (C), 135.7 (CH), 144.8 (C), 151.5 (C), 163.3 (C), 171.6 (C). EI-MS: 237 (M+, 12), 160 (15), 146 (18), 119 (44), 92 (100), 77 (58).
2.3.8. 2-((2-Nitrophenyl)amino)quinazolin-4(1H)-one (9h)
Gray powder; yield: 0.24 g (84%); mp: 181 - 183°C. IR (KBr) (νmax, cm-1): 3521, 3341, 1681, 1625, 1556, 1346, 1019. 1H-NMR (500MHz, CDCl3): δH = 6.11 (1 H, s, NH), 7.29 - 7.34 (3 H, m, H6,8-quinazolinone, H4-phenyl), 7.47 (1 H, d, 3J = 7.9 Hz, H5-quinazolinone), 7.60 - 7.63 (2 H, m, H5,6-phenyl), 7.74 (1 H, t, 3J = 8.1 Hz, H7-quinazolinone), 8.04 (1 H, d, 3J = 7.9 Hz, H3-phenyl), 9.20 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 127.8 (CH), 128.8 (2 CH), 129.0 (CH), 130.1 (2 CH), 131.1 (CH), 132.3 (C), 135.6 (CH), 141.1 (C), 144.8 (C), 150.1 (C), 161.0 (C), 170.2 (C). EI-MS: 282 (M+, 5), 164 (12), 160 (50), 137 (52), 123 (100), 119 (70).
2.3.9. 2-((4-Nitrophenyl)amino)quinazolin-4(1H)-one (9i)
Brown powder; yield: 0.24 g (86%); mp: 175 - 177°C. IR (KBr) (νmax, cm-1): 3410, 3389, 1649, 1615, 1554, 1348, 1111. 1H-NMR (500MHz, CDCl3): δH = 6.05 (1 H, s, NH), 6.92 (1 H, t, 3J = 7.9 Hz, H6-quinazolinone), 7.03 (1 H, t, 3J = 7.9 Hz, H7-quinazolinone), 7.14 (2 H, d, 3J = 8.2 Hz, H2,6-phenyl), 7.22 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.32 (1 H, d, 3J = 7.9 Hz, H5-quinazolinone), 7.57 (2 H, d, 3J = 8.2 Hz, H3,5-phenyl), 8.63 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.7 (2 CH), 129.8 (CH), 129.9 (CH), 130.1 (2 CH), 133.1 (CH), 133.9 (C), 138.3 (C), 138.9 (C), 145.3 (C), 155.0 (C), 162.1 (C), 172.3 (C). EI-MS: 282 (M+, 10), 164 (19), 160 (42), 137 (25), 123 (100), 119 (42).
2.3.10. 2-((3-Nitrophenyl)amino)quinazolin-4(1H)-one (9j)
Green powder; yield: 0.23 g (83%); mp: 169 - 171°C. IR (KBr) (νmax, cm-1): 3421, 3315, 1687, 1658, 1554, 1327, 1113. 1H-NMR (500MHz, CDCl3): δH = 6.00 (1 H, s, NH), 7.02 (1 H, t, 3J = 7.9 Hz, H6-quinazolinone), 7.07 (1 H, t, 3J = 8.1 Hz, H7-quinazolinone), 7.38 - 7.43 (4 H, m, H5,8-quinazolinone, H5,6-phenyl), 7.74 (1 H, d, 3J = 7.9 Hz, H4-phenyl), 7.91 (1 H, s, H2-phenyl), 8.48 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 127.9 (CH), 128.2 (CH), 128.3 (CH), 129.0 (CH), 129.3 (CH), 129.7 (CH), 129.8 (C), 130.1 (CH), 130.7 (CH), 140.1 (C), 147.2 (C), 155.2 (C), 167.2 (C), 174.3 (C). EI-MS: 282 (M+, 12), 164 (25), 160 (54), 137 (59), 123 (100), 119 (66).
2.3.11. 2-((4-Bromophenyl)amino)quinazolin-4(1H)-one (9k)
White powder; yield: 0.27 g (85%); mp: 164 - 166°C. IR (KBr) (νmax, cm-1): 3524, 3245, 1656, 1627, 1124. 1H-NMR (500MHz, CDCl3): δH = 5.85 (1 H, s, NH), 6.89 (1 H, t, 3J = 7.9 Hz, H6-quinazolinone), 7.12 (2 H, d, 3J = 7.8 Hz, H2,6-phenyl), 7.23 - 7.35 (3 H, m, H3,5-phenyl, H7-quinazolinone), 7.56 (2 H, d, 3J = 7.8 Hz, H5,8-quinazolinone), 8.53 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.6 (CH), 127.2 (CH), 129.7 (2 CH), 129.8 (2 CH), 130.0 (CH), 131.1 (C), 133.0 (CH), 138.3 (C), 139.0 (C), 145.2 (C), 160.0 (C), 172.0 (C). EI-MS: 315 (M+, 3), 196 (55), 160 (33), 155 (69), 119 (100).
2.3.12. 2-((3,4-Dichlorophenyl)amino)quinazolin-4(1H)-one (9l)
White powder; yield: 0.27 g (90%); mp: 191 - 193°C. IR (KBr) (νmax, cm-1): 3356, 3309, 1685, 1672, 1015. 1H-NMR (500MHz, CDCl3): δH = 6.03 (1 H, s, NH), 6.87 (1 H, t, 3J = 7.9 Hz, H6-quinazolinone), 7.03 (1 H, s, H2-phenyl), 7.11 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.18 (1 H, t, 3J = 7.9 Hz, H7-quinazolinone), 7.27 (1 H, d, 3J = 7.9 Hz, H6-phenyl), 7.29 (1 H, d, 3J = 8.3 Hz, H5-phenyl), 7.55 (1 H, d, 3J = 8.3 Hz, H5-quinazolinone), 8.52 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.7 (CH), 129.7 (2 CH), 129.8 (2 CH), 130.1 (C), 133.0 (CH), 138.3 (2 CH), 139.0 (C), 140.0 (C), 145.3 (C), 162.9 (C), 172.9 (C). EI-MS: 305 (M+, 1), 186 (25), 160 (23), 159 (64), 146 (100), 119 (32).
2.3.13. 2-((4-Fluorophenyl)amino)quinazolin-4(1H)-one (9m)
Cream powder; yield: 0.20 g (79%); mp: 143 - 145°C. IR (KBr) (νmax, cm-1): 3547, 3399, 1651, 1627, 1019. 1H-NMR (500MHz, CDCl3): δH = 6.05 (1 H, s, NH), 7.28 (1 H, t, 3J = 7.9 Hz, H6-quinazolinone), 7.30 - 7.35 (3 H, m, H3,5-phenyl, H7-quinazolinone), 7.36 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.50 (2 H, d, 3J = 7.8 Hz, H2,6-phenyl), 7.92 (1 H, d, 3J = 7.9 Hz, H5-quinazolinone), 8.53 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.2 (2 CH), 127.5 (CH), 127.7 (2 CH), 128.7 (CH), 129.2 (CH), 130.7 (C), 132.6 (CH), 142.2 (C), 147.2 (C), 152.2 (C), 160.2 (C), 172.2 (C). EI-MS: 255 (M+, 11), 160 (20), 146 (21), 137 (62), 110 (100), 96 (25).
2.3.14. 2-((4-Chlorophenyl)amino)quinazolin-4(1H)-one (9n)
White powder; yield: 0.23 g (84%); mp: 160 - 162°C. IR (KBr) (νmax, cm-1): 3425, 3308, 1671, 1625, 1025. 1H-NMR (500MHz, CDCl3): δH = 5.85 (1 H, s, NH), 6.92 (1 H, t, 3J = 7.9 Hz, H6-quinazolinone), 7.17 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.23 (1 H, d, 3J = 7.8 Hz, H5-quinazolinone), 7.35 (1 H, t, 3J = 7.9 Hz, H7-quinazolinone), 7.56 - 7.60 (4 H, m, H2,3,5,6-phenyl), 8.53 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.8 (2 CH), 129.3 (2 CH), 129.8 (CH), 130.2 (CH), 130.4 (CH), 132.6 (C), 133.2 (CH), 142.2 (C), 145.4 (C), 148.0 (C), 160.0 (C), 170.2 (C). EI-MS: 273 (M+2, 5), 271 (11), 153 (25), 146 (24), 126 (60), 119 (100), 112 (44).
2.3.15. 2-((3-Chlorophenyl)amino)quinazolin-4(1H)-one (9o)
White powder; yield: 0.21 g (78%); mp: 153 - 155°C. IR (KBr) (νmax, cm-1): 3448, 3325, 1685, 1627, 1015. 1H-NMR (500MHz, CDCl3): δH = 6.05 (1 H, s, NH), 7.26 (1 H, t, 3J = 7.9 Hz, H6-quinazolinone), 7.28 - 7.35 (3 H, m, H2,4,6-phenyl), 7.51 (1 H, d, 3J = 7.9 Hz, H8-quinazolinone), 7.62 (1 H, t, 3J = 7.9 Hz, H5-phenyl), 7.74 (1 H, t, 3J = 7.9 Hz, H7-quinazolinone), 8.05 (1 H, d, 3J = 7.9 Hz, H5-quinazolinone), 8.88 (1 H, s, NH). 13C-NMR (125.7MHz, CDCl3): 126.2 (2 CH), 127.8 (CH), 128.7 (CH), 129.2 (CH), 130.0 (CH), 130.1 (2 CH), 132.5 (C), 135.7 (C), 144.9 (C), 155.1 (C), 166.2 (C), 174.9 (C). EI-MS: 273 (M+2, 7), 271 (15), 153 (28), 146 (20), 126 (68), 119 (100), 112 (40).
2.4. Biological Evaluations
The biological activities of the synthesized compounds, 8a-o and 9a-o, were carried out using EGFR-TK assay kit (BPS biosciences) and kinase-Glo luminescent assay (Promega). In order to start the enzymatic reaction, EGFRT790M and their substrates were incubated with the synthesized compounds in kinase assay buffer for 60 min at room temperature. Then detection reagent (ADP-GloTM reagent) was added to each well, followed by incubation at room temperature for 40 min. With the addition of kinase detection reagent, the mixture was incubated for further 30 min. The IC50 values were determined after the luminescence record, and all samples and controls were tested in triplicate.
2.5. Docking Study
Crystallographic structure of EGFR tyrosine kinase was retrieved from Protein Data Bank (PDB code: 4G5P, resolution 3.17 Å). The docking analysis was performed using AutoDock Tools version 1.5.6rc3 (http://mgltools.scripps.edu) software to evaluate the binding mode of the compound 8b against EGFRT790M. At first, the original ligand and water molecules were removed, and polar hydrogen atoms and Kollman united partial atom charges were added onto the protein. The 2D structure of the synthesized compound and erlotinib were sketched using ChemBioDraw Ultra 12.0 and optimized by MM+ force field using HyperChem8 (http://www.hyper.com). Each docking system was performed by 100 runs of the Autodock using the Lamarckian genetic algorithm (LGA). The lowest docking-energy conformation of the highest populated cluster was considered the most stable orientation and selected for analysis. Finally, a cluster analysis was performed on the docking results using a root mean square deviation (RMSD) of 0.5 Å. Graphic manipulations and visualizations were done by Pymol software version 1.5.0.1 (http://pymol.findmysoft.com).
2.6. ADME Properties
The ADME properties of the synthesized compounds were predicted using the Molinspiration online property calculation toolkit (Molinspiration, 2018). Topological polar surface area (TPSA), number of rotatable bonds (n-ROTB), molecular volume (MV), molecular weight (MW), the logarithm of the partition coefficient (miLog P), number of hydrogen bond acceptors (n-ON), number of hydrogen bond donors (n-OHNH), and Lipinski's rule of five were calculated (23). Additionally, intestinal absorption (% ABS) was calculated according to the following equation: ABS% = 109 − (0.345 × TPSA) (24).
3. Results and Discussion
3.1. Chemistry
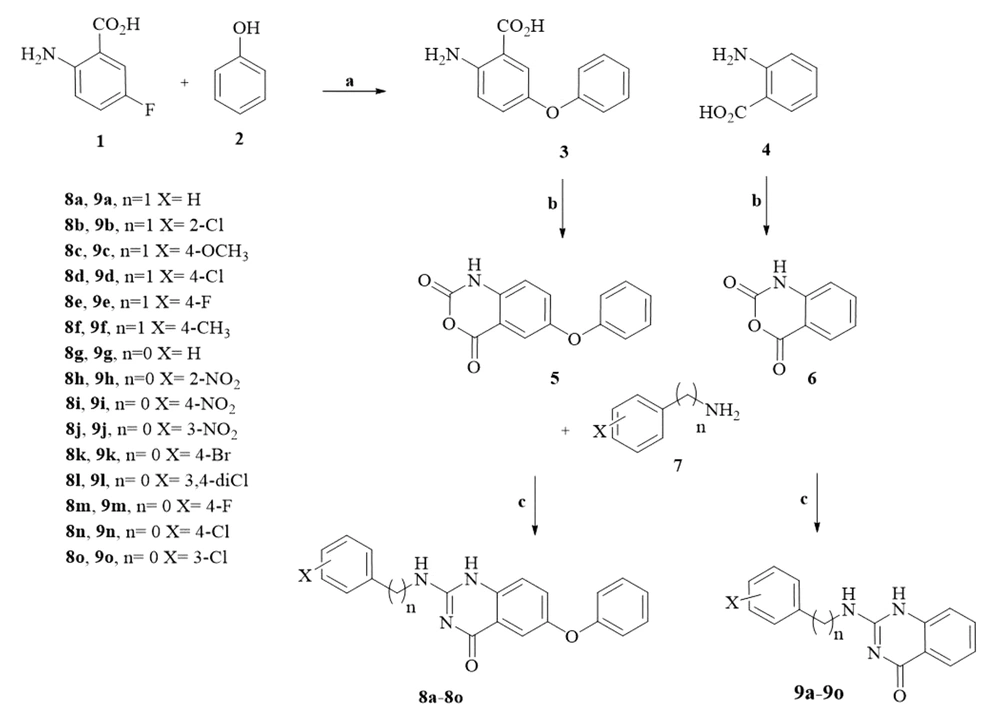
Figure 3 displayed the general synthetic pathway of the novel compounds. 2-Amino-5-fluorobenzoic acid 1 was reacted with phenol 2 through a nucleophilic aromatic substitution using sodium hydride as the base under reflux conditions to produce compound 3. Isatoic anhydride derivatives (5, 6) were achieved by reacting the compounds 3 and 2-aminobenzoic acid 4 with ethyl chloroformate and triethylamine. The final products 8(a-o) and 9(a-o) were afforded by the treatment of the above isatoic anhydrides with corresponding aniline or benzylamine in the presence of trichloroacetonitrile and catalytic amount of CuO under reflux condition (25).
3.2. Biological Evaluations
The inhibitory activities of the novel compounds were assessed using EGFRT790M/ADP-GloTM kinase assay system. Erlotinib, a reversible EGFR-TKI, was used as a standard compound. As shown in Figure 4, most synthesized compounds provided nanomolar range inhibition against EGFR-TK. Some derivatives of compound 8 (i.e., compounds 8a, 8b, 8c, 8d and 8f) exhibited higher inhibitory activities than the other compounds, compared to erlotinib (IC50 = 1.5 nM). The most potent compound was 8b, with an IC50 value of 1.37 nM. By comparing the IC50 values of compounds 8b and 9b, it can be concluded that introducing a phenoxy group at the 6-position could be beneficial for the activity owing to the proper fitting of the compound into a hydrophobic pocket. According to compounds 8c, 8f, and 9c, the presence of the electron-donating substituents in para position of benzyl ring in phenoxy series were more desirable than electron-withdrawing substituents; however, this finding cannot be extended to ortho position as compound 8b exhibited the highest activity with an electron withdrawing substituent. Substitution of fluorine in para position of either benzyl or phenyl ring (i.e., compounds 8m and 9m) was not favorable for the activity since fluorine, a hydrophilic atom could be surrounded by water molecules, leading to a difficult location into the hydrophobic pocket of the enzyme.
Moreover, compounds 8j and 9j possessing 3-nitro substituent on phenyl ring were potent analogs in both series. It could be explained that the nitro group could afford a hydrogen bond with polar amino acids in the active site. Generally, due to close variations of IC50s, the judgment about the activity trend was a challenge we were encountered, and we did not examine electron-donating substituents in phenyl bearing series, which is a limitation for our study.
3.3. Docking Study
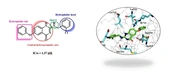
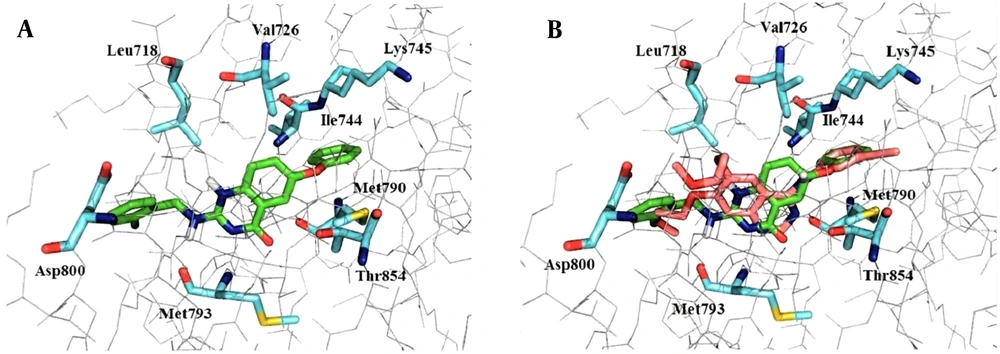
A docking study was performed to identify the binding mode of compound 8b and EGFR-TK active site. As shown in Figure 5, docking study revealed that this structure had a good affinity to the active site of the enzyme and fitted properly. The amide group of quinazolinone moiety and nitrogen in the hydrophobic head had suitable distances from Met793 for hydrogen bonding interaction. Additional hydrogen bonds formed between the oxygen of the phenoxy group and residues of Met790 and Thr854. As compound 8b was surrounded by lipophilic amino acids, hydrophobic interactions were observed between 8b and Lys745, Val726, Leu718, Ile744. Furthermore, the superimposition of compound 8b and erlotinib demonstrated that these two structures were located in the same direction in the active site as the nitrogen atoms at the 1-position of erlotinib and 3-position of 8b were completely aligned and formed hydrogen bonds with Met793.
(A) Compound 8b (green sticks) in the active site of EGFRT790M receptor (amino acid residues in blue PDB: 4G5P). The amide group of quinazolinone moiety and nitrogen in the hydrophobic head had suitable distances from Met793 for hydrogen bonding interaction. Additional hydrogen bond formed between oxygen of phenoxy group and Met790 and Thr854. The hydrophobic interactions observed between 8b and Lys745, Val726, Leu718, and Ile744. (B) The superimposition of compound 8b (green sticks) and erlotinib (salmon pink sticks) in the active site of EGFRT790M receptor.
3.4. ADME Properties
To predict oral bioavailability and druglikeness of our novel compounds, ADME calculations were performed using molinspiration calculator (Table 1). According to the Lipinski's rule of five (miLog P (octanol–water partition coefficient) ≤ 5, molecular weight ≤ 500, number of hydrogen bond acceptors ≤ 10, and number of hydrogen bond donors ≤ 5), an orally active drug candidate has no more than one violation of the criteria (26). All the synthesized compounds exhibited an excellent ABS% ranging from 70.07% to 89.06%. They could be orally active agents through fulfilling the criteria.
| Number | %ABS | TPSA (A2) | n ROTB | MV | MW | miLogP | n-ON Acceptors | n-OHNH Donors | Lipinski's Violations |
|---|---|---|---|---|---|---|---|---|---|
| 8a | 85.88 | 67.02 | 5 | 308.85 | 343.39 | 4.71 | 5 | 2 | 0 |
| 8b | 85.88 | 67.02 | 5 | 322.39 | 377.83 | 5.34 | 5 | 2 | 1 |
| 8c | 82.69 | 76.25 | 6 | 334.40 | 373.41 | 4.77 | 6 | 2 | 0 |
| 8d | 85.88 | 67.02 | 5 | 322.39 | 377.83 | 5.39 | 5 | 2 | 1 |
| 8e | 85.88 | 67.02 | 5 | 313.79 | 361.38 | 4.88 | 5 | 2 | 0 |
| 8f | 85.88 | 67.02 | 5 | 325.42 | 357.41 | 5.16 | 5 | 2 | 1 |
| 8g | 85.88 | 67.02 | 4 | 292.05 | 329.36 | 5.03 | 5 | 2 | 1 |
| 8h | 70.07 | 112.84 | 5 | 315.39 | 374.36 | 4.94 | 8 | 2 | 0 |
| 8i | 70.07 | 112.84 | 5 | 315.39 | 374.36 | 4.99 | 8 | 2 | 0 |
| 8j | 70.07 | 112.84 | 5 | 315.39 | 374.36 | 4.96 | 8 | 2 | 0 |
| 8k | 85.88 | 67.02 | 4 | 309.94 | 408.25 | 5.84 | 5 | 2 | 1 |
| 8l | 85.88 | 67.02 | 4 | 319.12 | 398.25 | 6.31 | 5 | 2 | 1 |
| 8m | 85.88 | 67.02 | 4 | 296.98 | 347.35 | 5.19 | 5 | 2 | 1 |
| 8n | 85.88 | 67.02 | 4 | 305.59 | 363.80 | 5.71 | 5 | 2 | 1 |
| 8o | 85.88 | 67.02 | 4 | 305.59 | 363.80 | 5.68 | 5 | 2 | 1 |
| 9a | 89.06 | 57.78 | 3 | 228.46 | 251.29 | 2.98 | 4 | 2 | 0 |
| 9b | 89.06 | 57.78 | 3 | 242.00 | 285.73 | 3.61 | 4 | 2 | 0 |
| 9c | 85.88 | 67.02 | 4 | 254.01 | 281.31 | 3.04 | 5 | 2 | 0 |
| 9d | 89.06 | 57.78 | 3 | 242.00 | 285.73 | 3.66 | 4 | 2 | 0 |
| 9e | 89.06 | 57.78 | 3 | 233.39 | 269.28 | 3.15 | 4 | 2 | 0 |
| 9f | 89.06 | 57.78 | 3 | 245.02 | 265.32 | 3.43 | 4 | 2 | 0 |
| 9g | 89.06 | 57.78 | 2 | 211.66 | 237.26 | 3.30 | 4 | 2 | 0 |
| 9h | 73.25 | 103.61 | 3 | 234.99 | 282.26 | 3.21 | 7 | 2 | 0 |
| 9i | 73.25 | 103.61 | 3 | 234.99 | 282.26 | 3.26 | 7 | 2 | 0 |
| 9j | 73.25 | 103.61 | 3 | 234.99 | 282.26 | 3.23 | 7 | 2 | 0 |
| 9k | 89.06 | 57.78 | 2 | 229.54 | 316.16 | 4.11 | 4 | 2 | 0 |
| 9l | 89.06 | 57.78 | 2 | 238.73 | 306.15 | 4.58 | 4 | 2 | 0 |
| 9m | 89.06 | 57.78 | 2 | 216.59 | 255.25 | 3.46 | 4 | 2 | 0 |
| 9n | 89.06 | 57.78 | 2 | 225.19 | 271.71 | 3.98 | 4 | 2 | 0 |
| 9o | 89.06 | 57.78 | 2 | 225.19 | 271.71 | 3.95 | 4 | 2 | 0 |
| Erlotinib | 83.31 | 74.45 | 10 | 362.06 | 393.44 | 2.79 | 7 | 1 | 0 |
Abbreviations: ABS%, percentage absorption; TPSA, topological polar surface area; n-ROTB, number of rotatable bonds; MV, molecular volume; MW, molecular weight; miLog P, logarithm of partition coefficient of the compound between n-octanol and water; n-ON acceptors, number of hydrogen bond acceptors; n-OHNH donors, number of hydrogen bonds donors.
4. Conclusions
In summary, we designed and synthesized thirty qinazolinone derivatives as EGFR-TKIs. All the compounds were evaluated for their inhibitory activities against EGFRT790M. Most of our novel compounds provided nanomolar range inhibition. Compounds 8a, 8b, 8c, 8d, and 8f exhibited more potent inhibitory activities than erlotinib. Remarkably, Compound 8b with 2-chlorobenzyl group in phenoxy derivatives showed the exemplary inhibitory activity with an IC50 value of 0.37 nM. The docking study of 8b demonstrated that it had a good affinity to the active site of the enzyme and fitted properly. According to our findings, attaching a phenoxy group at the 6-position could be beneficial for the activity due to the proper disposition of the compound into the hydrophobic pocket of the enzyme. Based on ADME prediction analysis, the synthesized compounds possessed excellent druglikeness properties to be formulated for the oral route of administration. The extension of this work would provide valuable facts for the further design of EGFR-TKIs.