1. Background
The human immunodeficiency virus (HIV) represents one of the significant health challenges of the world, with a substantial social and economic impact on public health (1-3). Based on the latest report of the Joint United Nations Program on HIV/AIDS (UNAIDS), 38.0 million patients globally were living with HIV in 2019, and in the same year, 1.7 million people became newly infected with HIV (4). That is why it is still crucial to find new ways to effectively combat the disease. The currently used highly active antiretroviral therapy (HAART) is reported as the most effective anti-HIV drug regimen that significantly decreases HIV viral load, changing AIDS from a rapidly lethal condition to a manageable chronic disease (5-8). Nevertheless, HAART benefits are still overshadowed by problems such as drug-drug interactions, adverse drug reactions, poor patient adherence, high costs, and the emergence of drug-resistant viral strains (9-11).
For this reason, trying to find novel anti-HIV medicines with improved efficacy and less toxicity is still ongoing (12, 13). On the other hand, the use of designed multiple ligands (DMLs), which are single scaffolds with multiple biological targets, is a growing endeavor in medicinal chemistry (14, 15). Reverse transcriptase (RT), integrase (IN), and protease (PR) are three essential HIV enzymes that are targeted by the commonly-used anti-HIV drugs, including RT, PR, and IN inhibitors (1, 16). A new group of DMLs can be developed by modeling the chemical structures of these three classes and combining their main pharmacophores (17). A group of antiretroviral drugs that bind to the RT p66 subunit in a hydrophobic pocket away from the enzyme catalytic site is non-competitive Non-nucleoside RT inhibitors (NNRTIs) (18, 19). Such molecules here assume a kind of “butterfly” or “horseshoe” conformation that induces a conformational change in the enzyme and therefore limits its activity (20-25). HIV protease inhibitors (PIs) are another group of anti-HIV agents that were once the mainstay of HIV treatment. Most of the peptidomimetic PIs contain a non-hydrolyzable hydroxy ethylene core that prevents the cleavage of the precursor polyprotein, needed for the formation of the mature viral proteins (21, 23). IN inhibitors, a relatively newer class of anti-HIV agents commonly contain a structural motif that coordinates two Mg2+ ions present in the enzyme’s active site, by which the enzyme activity is increased. They are expected to demonstrate a low frequency of adverse effects since no homolog for the enzyme exists in humans (26, 27). Some of the HIV-inhibitors from different classes are depicted in Figure 1. Herein, a novel series of 2-(diphenyl methylidene) malonic acid derivatives were developed as multiple-action HIV RT/IN/PR inhibitors. The presence of symmetric lipophilic moieties with a butterfly conformation in all of the newly-synthesized compounds has been presumed to be responsible for RT/PR inhibition activity, as it was previously described (28-31). Furthermore, as mentioned above, the structure of INIs consists of a chelating motif to sequestrate one or both divalent metal ions at the enzyme catalytic core. Considering several earlier studies, the heterocyclic oxadiazole ring can perform as a chelating motif (32-34). Moreover, it is anticipated that the rest of the compounds, which have heteroatoms with an acceptable distance and specific spatial arrangement, can adequately coordinate the Mg2+ ions, as well. Docking studies were also performed to forecast the binding mode of newly synthesized compounds with the binding sites of the target enzymes and their probable mechanism of action. Finally, the anti-HIV activity of these compounds was evaluated against the HIV virus (NL4-3) in HeLa cells cultures.
2. Methods
2.1. Chemistry
2.1.1. Materials and Methods
All chemicals were purchased from the Sigma-AldrichTM (USA) and MerckTM (Germany) chemical companies with a minimum purity of 97% and used without further purification. Reaction’s progress was envisioned by thin-layer chromatography (TLC) performed on pre-coated silica gel plates (MerckTM, Kieselgel 60 F-254, 0.2 mm). Melting points were determined on the Electrothermal-9100 apparatus and were uncorrected. The structures of the novel compounds were confirmed by IR, LC/MS, 1H-NMR, 13C-NMR, and elemental analysis. Infrared (IR) spectra were acquired on a Perkin-ElmerTM 843 IR spectrometer. BrukerTM advance II (100, 125, 400, 500 MHz) spectrophotometer (BrukerTM Biosciences, USA) obtained NMR spectra at ambient temperature using TMS as the internal standard and d6-DMSO or CDCl3 as the solvent. The NMR spectra were recorded in ppm using automatic calibration to tetramethylsilane (TMS) as the internal standard. The 1H NMR data were presented as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, dd = doublet of doublet, t = triplet, q = quartet, m = multiplet), coupling constants (Hz) and integration. The 13C-NMR analyzes were reported in terms of chemical shift (δ ppm). The mass spectrophotometry was carried out on a 6410 Agilent LC-MS triple quadrupole mass spectrometer (LC-MS) equipped with an electrospray ionization (ESI) interface. An elemental analyzer (CostechTM, Italy) was also used to carry out the elemental analysis. Purification of the compounds was performed with the specified eluent using silica gel column chromatography (230 - 400 mesh size).
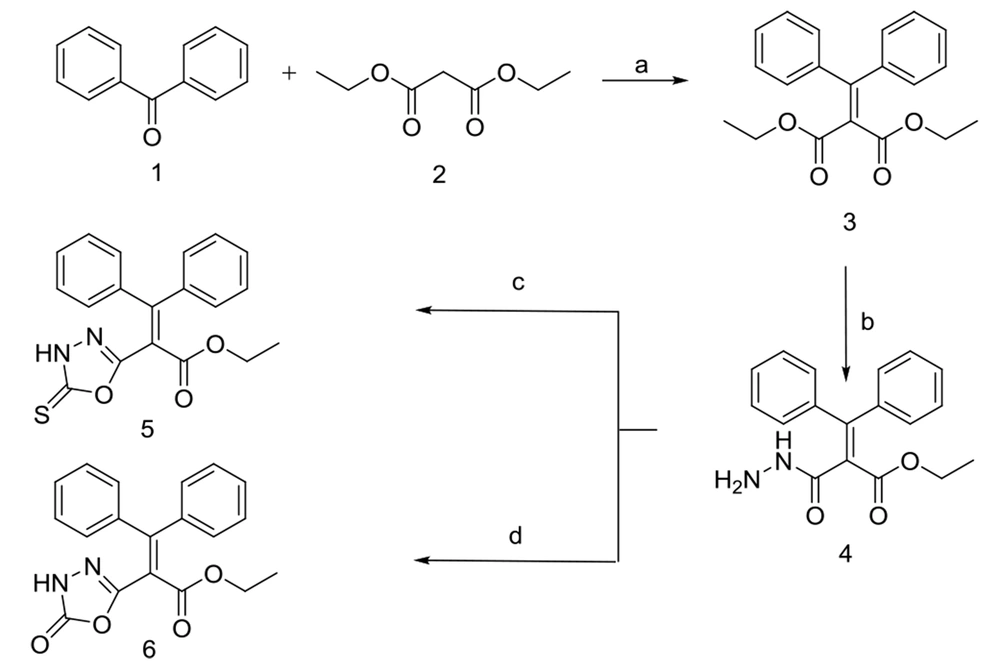
2.1.1.1. Preparation of Diethyl2-(Diphenyl Methylene) Malonate (3)
TiCl4 (12.05 mL, 109.6 mmol) was added gradually to dry THF (110 mL), and the reaction flask was placed in an ice bath. Afterward, benzophenone 1 (5 g, 27.4 mmol) and diethyl malonate 2 (10.5 mL, 68.5 mmol) were added. Subsequently, dry pyridine (17.7 mL, 219.2 mmol) was added to the reaction mixture dropwise in 0.5 to 1 h and stirred at 25°C for 48 h. The crude residue was dispersed in 25 mL water and extracted with diethyl ether upon the reaction completion. The organic layer was shaken with a saturated NaHCO3 aqueous solution, then with brine, and finally dried over anhydrous Na2SO4. Silica gel column chromatography eluting with EtOAc/n-hexane (0.5: 9.5) was utilized to give the purified compound 3.
Colorless crystals, yield: 67%; mp 73.5 - 75°C; 1HNMR (500 MHz, DMSO-d6,) (δ, ppm): 0.88 (t, J = 7.09 Hz, 6H, CH3), 3.95 (q, J = 7.1 Hz, 4H, OCH2), 7.07 (dd, J = 6.43 Hz, 1.65 Hz, 4H, Ar-H), 7.32-7.39 (m, 6H, Ar-H); 13CNMR (125 MHz, DMSO-d6) (δ, ppm): 14.27, 61.77, 126.94, 129.21, 129.55, 130.13, 140.39, 155.32, 166.06; ESI-MS (m/z): 347 [M + Na]+, 671 [2M + Na]+; IR (KBr disk) ν (cm-1): 1760, 1773 (C = O), 1450-1600 (aromatic). Anal. Calcd. for C20H20O4: C, 74.06; H, 6.21. Found: C, 74.11; H, 6.19.
2.1.1.2. Preparation of Ethyl 2-(Hydrazinecarbonyl)-3,3-Diphenylacrylate (4)
Compound 3 was dissolved in DMF (0.2 mL) and treated with hydrazine hydrate (1: 5). The mixture was stirred for 18 hours at room temperature. Then, water was added into the reaction mixture, and the resulting precipitate was collected by filtration, boiled in ethanol 96%, and washed with n-hexane to afford compound 4.
White solid, yield: 67%; mp: 199-200 °C; 1HNMR (400 MHz, DMSO-d6) (δ, ppm): 0.92 (t, J = 7.2 Hz, 3H, CH3), 3.95 (m, 2H, OCH2), 4.20 (s, 1H, NH), 6.04 (s, 1H, NH), 7.21 (dd, 7.2 Hz, 2H, Ar-H), 7.28 - 7.37 (m, 6H, Ar-H), 7.74 (d, J = 7.2 Hz, 2H, Ar-H), 9.96 (s, 1H, NH); 13CNMR (100 MHz, DMSO-d6) (δ, ppm): 14.01, 59.44, 61.33, 73.93, 125.74, 126.52, 127.62, 127.72, 128.87, 128.92, 142.05, 144.31, 168.14, 172.11; ESI-MS (m/z): 311 [M + H]+, 333 [M + Na]+, 349 [M + K]+; IR (KBr disk) ν (cm-1): 3117, 3188, 3256 (N-H), 1702, 1740 (C = O), 1450 - 1600 (aromatic). Anal. Calcd. for C18H18N2O3: C, 69.66; H, 5.85; N, 9.03. Found: C, 69.61; H, 5.84; N, 9.06.
2.1.1.3. Preparation of Ethyl 3,3-Diphenyl-2-(5-Thioxo-4,5-Dihydro-1,3,4-Oxadiazol-2-yl) Acrylate (5)
Compound 4 (1 g, 3.23 mmol) was dissolved in dry DMF, and 0.4 g KOH (7.13 mmol) and then 2.3 mL CS2 (38.71 mmol) were added dropwise to the solution while the reaction flask was placed into an ice bath. The reaction mixture was stirred for 48 hours at 80 °C. The resulting solution was cooled to room temperature and acidified with HCl (2N). The precipitate was filtered and washed entirely with distilled water. The crude residue was subjected to column chromatography (silica gel, hexane/EtOAc, 6.5:3.5) to afford the pure compound 5.
Yellow solid, yield: 30%; mp: 95-97 °C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 0.83 (t, J = 7.06 Hz, 3H, CH3), 3.90 (q, J = 7.09 Hz, 2H, OCH2), 6.95 (d, J = 6.85 Hz, 2H, Ar-H), 7.07 (t, J = 3.61 Hz, 2H, Ar-H), 7.24-7.34 (m, 6H, Ar-H); 13CNMR (125 MHz, DMSO-d6) (δ, ppm): 14.25, 61.68, 119.73, 128.95, 129.16, 129.50, 129.60, 130.24, 140.68, 141.66, 150.59, 159.34, 167.35, 180.73; ESI-MS (m/z): 353 [M + H]+; IR (KBr disk) ν (cm-1): 1711 (C = O), 1450 - 1600 (aromatic). Anal. Calcd. for C19H16N2O3S: C, 64.76; H, 4.58; N, 7.95; Found: C, 64.74; H, 4.60; N, 7.98.
2.1.1.4. Preparation of Ethyl 2-(5-oxo-4,5-Dihydro-1,3,4-Oxadiazol-2-yl)-3,3-Diphenylacrylate (6)
Compound 4 (1 g, 3.23 mmol) was dissolved in dry THF, while the reaction flask was placed into an ice bath. Triethylamine (2.6 mL, 18.52 mmol) was added, and the mixture was stirred for 2 minutes. Then 1, 1’-Carbonyldiimidazole (3.2 g, 19.73 mmol) was added and stirred continuously for 28 hours. The mixture was then filtered. The subfiltrate was acidified with HCl 20% till pH 2 - 3, and the organic layer was extracted by EtOAc. Finally, the solvent was evaporated under vacuo, and the residue was subjected to column chromatography (silica gel, n-hexane/ EtOAc, 6.5: 3.5) to obtain the purified compound 6.
White solid, yield: 20%; mp: 115 - 117°C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 0.83 (br, 3H, CH3), 3.91 (br, 2H, OCH2), 6.80 - 7.80 (m, 10 H, Ar-H); 13CNMR (100 MHz, DMSO-d6) (δ, ppm): 14.02, 56.21, 117.75, 125.77, 127.05, 128.06, 128.39, 129.06, 130.00, 137.47, 139.67, 142.14, 152.42, 156.05, 165.60, 167.71; ESI-MS (m/z): 337 [M + H]+, 359 [M + Na]+; IR (KBr disk) ν (cm-1): 1720, 1775 (C = O), 1684 (C = N), 1450-1600 (aromatic). Anal. Calcd. for C19H16N2O4: C, 67.85; H, 4.79; N, 8.33. Found: C, 67.80; H, 4.78; N, 8.34.
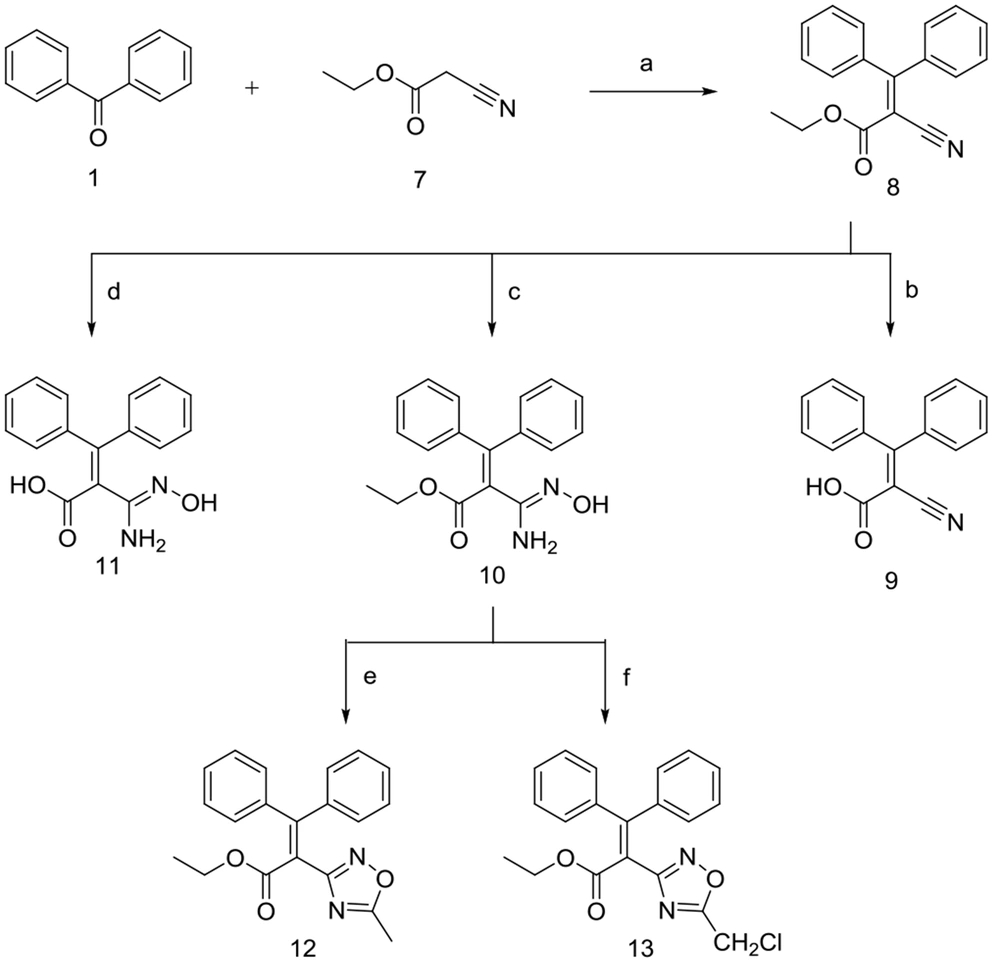
2.1.1.5. Preparation of Ethyl 2-Cyano-3,3-Diphenylacrylate (8)
TiCl4 (14.5 mL, 132 mmol) was gradually added to dry THF (90 mL) while the reaction flask was placed in an ice bath. Afterward, benzophenone 1 (6 g, 33 mmol) and ethyl cyanoacetate 7 (17.6 mL, 165 mmol) were added. Consequently, dry pyridine (21.2 mL, 263 mmol) was added to the reaction mixture dropwise in 0.5 to 1 h. The reaction mixture was stirred for 48 h at room temperature. The crude residue was dispersed in 30 mL water and extracted with diethyl ether upon the reaction completion. The organic layer was washed with a saturated NaHCO3 aqueous solution, then with brine, and finally dried over anhydrous Na2SO4. The resulting precipitate was collected by filtration, washed with distilled water, then with n-hexane, and subsequently dried under vacuum to afford compound 8.
Yellow solid, yield: 71%; mp: 73 - 75°C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 0.94 (t, J = 7.09 Hz, 3H, CH3), 4.02 (q, J = 7.08 Hz, 2H, OCH2), 7.12 (d, J = 7.46 Hz, 2H, Ar-H), 7.35 - 7.39 (m, 4H, Ar-H), 7.43 - 7.46 (m, 3H, Ar-H), 7.51 (t, J = 7.26 Hz, 1H, Ar-H); 13CNMR (125 MHz, DMSO-d6) (δ, ppm): 14.25, 62.69, 104.94, 117.56, 129.11, 129.49, 129.87, 130.59, 131.13, 132.15, 139.17, 139.20, 162.92, 169.43; ESI-MS (m/z): 278 [M + H]+, 300 [M + Na]+, 577 [2M + Na]+; IR (KBr disk) ν (cm-1): 2202 (CN), 1706 (C = O), 1450-1600 (aromatic). Anal. Calcd. for C18H15NO2: C, 77.96; H, 5.45; N, 5.05. Found: C, 77.91; H, 5.49; N, 5.07.
2.1.1.6. Preparation of 2-Cyano-3, 3-Diphenylacrylic Acid (9)
A methanolic solution of NaOH (0.112 g, 2.88 mmol) was added to a solution of the compound 8 (0.4 g, 1.44 mmol), in a CH2Cl2/CH3OH (9:1, v/v) mixture. The mixture was stirred for 1.5 hours until all the starting material was consumed. The solvent was then removed under a vacuum. To the dried residue, water was added, and the resulting solution was extracted with diethyl ether. The aqueous layer was then cooled and acidified with dilute HCl till pH 2-3. The solid started precipitating, filtered to obtain the purified compound 9.
White solid, yield: 60%; mp: 209-210 °C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 7.14 (d, J = 7.13 Hz, 2H, Ar-H), 7.32-7.38 (m, 4H, Ar-H), 7.40-7.51 (m, 4H, Ar-H); 13CNMR (125 MHz, DMSO-d6) (δ, ppm): 106.28, 118.04, 129.02, 129.44, 129.91, 130.45, 130.93, 131.80, 139.28, 139.56, 164.13, 168.14; ESI-MS (m/z): 250 [M+H]+, 272 [M+Na]+; IR (KBr disk) ν (cm-1): 2526-3061 (O-H), 2218 (CN), 1680 (C = O), 1450-1600 (aromatic). Anal. Calcd. for C16H11NO2: C, 77.10; H, 4.45; N, 5.62. Found: C, 77.16; H, 4.44; N, 5.60.
2.1.1.7. Preparation of Ethyl 2-(N’-Hydroxycarbamimidoyl)-3,3-Diphenylacrylate (10)
To 3.76 g of hydroxylammonium chloride (54.1 mmol) and 5.74 g of Na2CO3 (54.1 mmol), 30 mL water was added, and the mixture was stirred for 10 min until it became a clear solution. Then a methanolic solution (30 mL) of compound 8 (3 g, 11 mmol) was added, and the mixture was stirred for 72 h at room temperature. The resulting precipitate was filtered and subjected to flash chromatography (silica gel, n-hexane/EtOAc, 8: 2) to afford the purified compound 10.
White solid, yield: 42%; mp: 121.7 - 123.7°C; 1HNMR (500 Hz, DMSO-d6) (δ, ppm): 1.01 (br, 3H, CH3), 3.95 (q, J = 6.99 Hz, 2H, OCH2), 7.19 - 7.33 (m, 12H, Ar-H, NH2), 7.89 (s, 1H, OH); 13CNMR (100 MHz, DMSO-d6) (δ, ppm): 14.49, 60.81, 120.81, 124.61, 125.06, 127.36, 127.94, 128.12, 128.81, 128.95, 129.16, 129.35, 129.58, 136.01, 139.31, 164.90, 168.39, 169.89; ESI-MS (m/z): 311 [M + H+], 333 [M + Na]+, 643 [2M + Na]+; IR (KBr disk) ν (cm-1): 3332, 3449 (N-H), 3100 - 3500 (O-H), 1680 (C = O), 1450 - 1600 (aromatic). Anal. Calcd. for C18H18N2O3: C, 69.66; H, 5.85; N, 9.03. Found: C, 69.70; H, 5.86; N, 9.00.
2.1.1.8. Preparation of 2-(N’-Hydroxycarbamimidoyl)-3,3-Diphenylacrylic Acid (11)
To 3.76 g of hydroxylammonium chloride (54.1 mmol) and 5.74 g of Na2CO3 (54.1 mmol), 30 mL water was added, and the mixture was stirred for 10 minutes until it became a clear solution. A methanolic solution (30 mL) of compound 8 (3 g, 11 mmol) was then added, and the mixture was stirred for 72 h at room temperature. The resulting precipitate was collected by filtration under a vacuum. The crude solid was further purified by flash chromatography (silica gel) eluted by n-hexane/EtOAc (8: 2) to yield the pure compound 11.
White solid, yield: 58%; mp: 134-136 °C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 7.24 (d, J = 6.89 Hz, 2H, Ar-H), 7.32 - 7.44 (m, 10 H, Ar-H, NH2), 11.30 (s, 1H, OH); 13CNMR (125 MHz, DMSO-d6) (δ, ppm): 127.82, 129.00, 129.20, 129.24, 129.69, 129.74, 134.40, 137.62, 156.00; ESI-MS (m/z): 283 [M + H]+; IR (KBr disk) ν (cm-1): 3273 (N-H), 2732 - 3535 (O-H), 1721 (C = O), 1450 - 1600 (aromatic). Anal. Calcd. for C16H14N2O3: C, 68.07; H, 5.00; N, 9.92. Found: C, 68.15; H, 5.01; N, 9.87.
2.1.1.9. Preparation of Ethyl 2-(5-Methyl-1,2,4-Oxadiazol-3-Yl)-3,3-Diphenylacrylate (12)
Compound 10 (0.32 g, 1.03 mmol) and 110.2 μL acetyl chloride (1.54 mmol) were added to 15 mL dry toluene and refluxed for 6 h in the presence of 0.25 mL dry pyridine. The solvent was vaporized, and the precipitate filtered. The crude residue was washed with water and then n-hexane to obtain compound 12.
White solid, yield: 47%; mp: 209.1 - 210.3°C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 0.74 (t, J = 7.06 Hz, 3H, CH3), 2.16 (s, 3H, CH3), 3.85 (q, J = 7.07 Hz, 2H, OCH2), 7.15 - 7.20 (m, 5H, Ar-H), 7.24 - 7.30 (m, 3H, Ar-H), 7.47 (d, J = 7.82 Hz, 2H, Ar-H); 13CNMR (125 MHz, DMSO-d6) (δ, ppm): 14.24, 23.65, 61.85, 119.21, 128.60, 129.59, 129.91, 130.25, 130.51, 130.71, 130.82, 138.28, 153.46, 163.01, 163.52, 164.13; ESI-MS (m/z): 335 [M + H]+, 669 [2M + H]+; IR (KBr disk) ν (cm-1): 1736 (C = O), 1678 (C = N), 1450-1600 (aromatic). Anal. Calcd. for C20H18N2O3: C, 71.84; H, 5.43; N, 8.38. Found: C, 71.79; H, 5.44; N, 8.40.
2.1.1.10. Preparation of Ethyl 2-(5-(Chloromethyl)-1,2,4-Oxadiazol-3-Yl)-3,3-Diphenylacrylate (13)
A solution of compound 10 (0.45 g, 1.45 mmol) in 20 mL dry toluene containing 139 μL of chloroacetyl chloride (1.74 mmol) and 0.35 mL dry pyridine was refluxed for 6 h. The mixture was vacuum-concentrated after the completion of the reaction. The crude residue was filtered and washed with water and n-hexane. Finally, compound 13 was obtained by recrystallization from methanol.
Purple solid, yield: 13.5%; mp: 141 - 144°C; 1HNMR (500 MHz, CDCl3) (δ, ppm): 0.52 (t, J = 7.15 Hz, 3H, CH3), 3.67 (q, J = 7.15 Hz, 2H, OCH2), 4.47 (s, 2H, CH2), 6.71 (d, J = 7.83 Hz, 2H, Ar-H), 7.03 (t, J = 7.25 Hz, 1H, Ar-H), 7.10 (t, J = 7.83 Hz, 2H, Ar-H), 7.18 (d, J = 7.33 Hz, 2H, Ar-H), 7.26 (t, J = 7.31 Hz, 2H, Ar-H), 7.33 (t, J = 7.18 Hz, 1H, Ar-H); 13CNMR (125 MHz, CDCl3) (δ, ppm): 13.44, 45.24, 61.00, 94.08, 125.24, 126.43, 128.77, 128.94, 129.26, 130.14, 135.55, 138.19, 167.38, 167.96, 169.08, 170.37; ESI-MS (m/z): 369, 371 (3: 1) [M + H]+, 391, 393 (3:1) [M + Na]+; IR (KBr disk) ν (cm-1): 1715 (C = O), 1632, 1659 (C = N), 1450 - 1600 (aromatic). Anal. Calcd. for C20H17ClN2O3: C, 65.13; H, 4.65; N, 7.60. Found: C, 65.21; H, 4.60; N, 7.62.
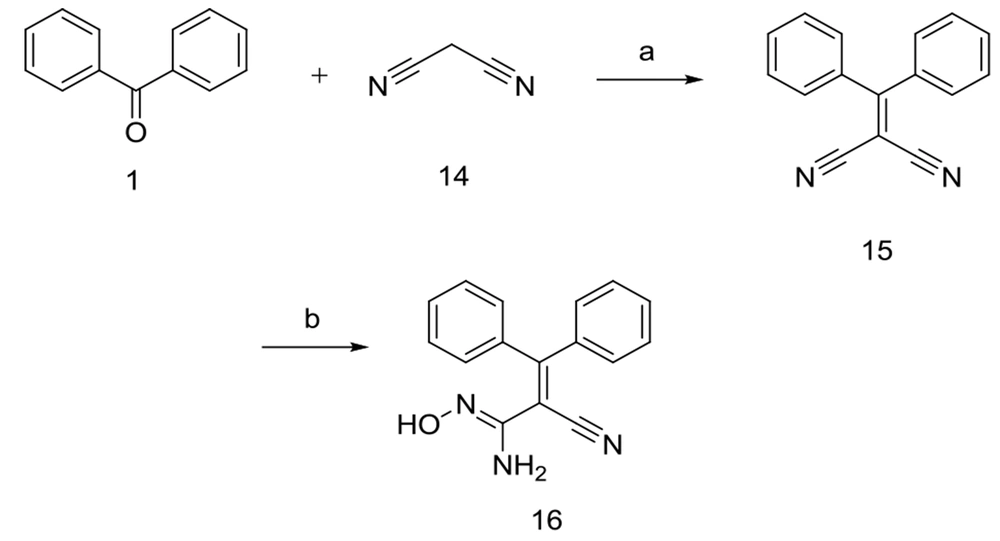
2.1.1.11. Preparation of 2-(Diphenyl Methylene) Malononitrile (15)
A mixture of benzophenone 1 (3 g, 16 mmol) and malononitrile 14 (1.63 g, 25 mmol) was stirred at 150°C for 1 h in the presence of ammonium acetate (1.27 g, 16 mmol). The mixture was permitted to cool down to 25°C after the reaction was completed. The solid was collected by filtration and washed with a minimum of distilled water and n-hexane. The purified compound 15 was recrystallized from ethanol 96%.
Yellow solid, yield: 32.5%; mp: 140.2 - 140.8°C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 7.45 (d, J = 7.57 Hz, 4H, Ar-H), 7.52 (t, J = 7.53 Hz, 4H, Ar-H), 7.60 (t, J = 7.19 Hz, 2H, Ar-H); 13CNMR (125 MHz, DMSO-d6) (δ, ppm): 82.96, 115.09, 129.72, 131.03, 133.40, 136.86, 175.10; ESI-MS (m/z): 231 [M + H]+, 253 [M + Na]+; IR (KBr disk) ν (cm-1): 2212 (CN), 1450 - 1600 (aromatic). Anal. Calcd. for C16H10N2: C, 83.46; H, 4.38; N, 12.17. Found: C, 83.52; H, 4.37; N, 12.15.
2.1.1.12. Preparation of 2-Cyano-N’-Hydroxy-3,3-Diphenylacrylimidamide (16)
A mixture of 2.26 g hydroxylammonium chloride (32 mmol) and 3.46 g Na2CO3 (32 mmol) in 25 mL water was stirred for 10 min until it became a clear solution. Then, a methanolic solution of compound 15 (1.5 g, 6.5 mmol) was added, and the mixture was stirred for 48 hours at room temperature. The resulting precipitate was collected by filtration under vacuum, washed with water and dried to afford compound 16.
Yellow solid, yield: 87.7%; mp: 128.1 - 128.7°C; 1HNMR (500 MHz, DMSO-d6) (δ, ppm): 7.25 - 7.28 (m, 2H, Ar-H), 7.33 - 7.34 (m, 8H, Ar-H), 7.38 (s, 2H, NH2), 8.28 (s, 1H, OH); 13CNMR (100 MHz, DMSO-d6) (δ, ppm): 76.31, 119,62. 120.69, 124.30, 125.71, 126.07, 127.44, 128.40, 129.06, 130.10, 131.23, 132.16, 137.47, 138.42, 166.83, 170.01; ESI-MS (m/z): 285 [M + Na]+, 549 [2M + Na]+; IR (KBr disk) ν (cm-1): 3331, 3399 (N-H), 2859 - 3633 (O-H), 2180 (CN). Anal. Calcd. for C16H13N3O: C, 72.99; H, 4.98; N, 15.96. Found: C, 72.87; H, 4.96; N, 15.98.
2.2. In-Vitro Anti-HIV Activity
The HIV inhibitory effects of the synthesized compounds were studied in vitro by a single cycle replication assay as detailed elsewhere (6, 35, 36). Briefly, HeLa cells were seeded at 6 × 103 cells per well in a 96-wells plate and infected with single-cycle replicable HIV NL4-3 virions (200 ng P24) concurrently with the addition of synthesized compounds in different levels (100 nm, 1, 10 and 100 μM). Seventy two hours after viral inoculation, the supernatants were collected and evaluated for P24 antigen load by capture ELISA (BiomerieuxTM, France). The inhibition rate (%) of P24 expression was calculated. Moreover, the cellular toxicity was evaluated by XTT (sodium 3-[1(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid) proliferation assay (RocheTM, Germany) according to the kit instruction (37, 38). The HIV replication assay plates were directly considered for cytotoxicity assay after determining the P24 load.
2.3. Molecular Modeling (Docking) Studies
The X-ray crystal structures of HIV PR (PDB ID: 1AJX), RT (PDB ID: 4KO0), and PFV IN (PDB ID: 3OYA) were retrieved from RCSB Protein Data Bank to serve as docking templates (39-41). All the compounds were sketched using ChemDraw® and subsequently energy-minimized using HyperChem 8.0. The protein structures were prepared for docking using Autodock tools 1.5.6 from MGL tools package (42), and docking was performed by AutoDock 4.2.3 software using a flexible ligand-rigid protein method (43). All water molecules were removed from the enzyme crystal structures, while Mg2+ ions were maintained at the active site of HIV IN. Polar hydrogens were added, and nonpolar hydrogens were merged. Finally, Kollman united atom charges, and the atom type parameter was assigned to the protein structures. Grid map dimensions (20 × 20 × 20) were set surrounding the active site. The Lamarckian genetic search algorithm was employed, and the docking run was set to 50. Finally, results were visualized by Pymol® (44) and Viewerlite® software.
3. Results and Discussion
3.1. Chemistry
The newly-designed compounds were synthesized with a wide range of yields (13.5 - 87%) according to Figures 2 - 4. Diester 3 was achieved through the well-known Knoevenagel reaction (45) of benzophenone 1 with diethyl malonate in the presence of dried pyridine and a catalytic amount of titanium chloride (TiCl4). Treatment of 3 with hydrazine hydrate in DMF afforded compound 4 via a process described before in the literature (46). Interestingly, according to spectral data, only one ester group of 3 was transformed into hydrazide, likely because of the formation of intramolecular hydrogen bonds, which decrease reactivity in the other ester group. However, more studies are required to justify this observation. Compounds 5 and 6 were obtained from the reaction of carbohydrazide 4 with carbon disulfide (CS2)/KOH (47) and triethylamine (TEA)/1,1’-carbonyldiimidazole (CDI) (48) during 48 and 28 h, respectively with further purification by column chromatography.
Ethyl 2-cyano-3,3-diphenyl acrylate 8, was prepared similarly, in which benzophenone 1 was reacted with ethyl cyanoacetate 7 in the presence of dry pyridine and a catalytic amount of titanium chloride. The ester 8 was subsequently hydrolyzed under mild non-aqueous alkaline conditions using dichloromethane/methanol (9: 1) as the solvent to obtain corresponding carboxylic acid 9.
Synthetic method B; Reagents and conditions: A, TiCl4, dry pyridine, dry THF, rt, 48 h; B, NaOH/methanol, CH2Cl2/methanol (9: 1), rt, 1.5 h; C and D, Hydroxylammonium chloride, sodium carbonate, methanol/H2O, rt, 72 h; E, Acetyl chloride, dry pyridine, dry toluene, reflux, 6 h; F, Chloroacetyl chloride, dry pyridine, dry toluene, reflux, 9.5 h.
Compound 8 was also reacted with hydroxylammonium chloride/Na2CO3, to give 10 and its hydrolyzed form 11, as shown in Figure 3. Lastly, compound 10 reacted with different acyl chlorides in the presence of pyridine and dry toluene to afford 1,2,4-oxadiazole analogs 12 and 13 in low to moderate yields.
In a separate set of reactions, benzophenone 1 reacted with malononitrile 14 in the presence of ammonium acetate to give the compound 15, which subsequently underwent further reaction with hydroxyl ammonium chloride and Na2CO3 in methanol/water mixture to afford the compound 16 in excellent yield (87.7 %).
Similar to the reaction of compound 3 with hydrazine hydrate, only one nitrile moiety of 15 converted to the amidoxime group. All compounds were structurally confirmed by analytical methods, including IR, ESI-Mass, 1H-NMR, 13C-NMR, and elemental analysis data.
3.2. Anti-HIV Activity
1, 1-Diphenyl ethene analogs with butterfly conformation have been previously reported as non-nucleoside RT/PR inhibitors (49, 50). In this study, diphenyl ethene moiety was combined with the essential pharmacophore to inhibit IN enzyme to synthesize a new group of anti-HIV drugs with multiple inhibitory actions against HIV enzymes. Herein, structure-activity relationship (SAR) studies around these lead compounds resulted in the identification of a series of analogs that demonstrated a broad range of HIV inhibition (8 - 300 μM), as outlined in Figure 5. All newly-synthesized molecules were assessed for their anti-HIV activity against the HeLa cells contaminated with single-cycle replicable HIV NL4-3 by determining their ability to inhibit p24 expression. A cell-based XTT colorimetric assay was simultaneously performed to ensure that the compounds’ anti-HIV activity was not the outcome of their cytotoxic effects (37, 51). Most of the novel compounds exhibited appropriate EC50 values compared to azidothymidine (AZT), the potent nucleoside RT inhibitor with EC50 values in nanomolar level against HIV-1 strain. The most potent compounds were lipophilic ethyl carboxylate and mercapto oxadiazole substituents on the methylene group (3 and 5). The reason may be that lipophilic groups connected with the diphenyl ethene form hydrophobic bond interactions with the binding site atoms and probably improve the cell membrane permeability as favored by anti-HIV activity. By contrast, the activity was dramatically decreased when ester was replaced by an ionizable carboxylic acid moiety (compounds 9 and 11), which might be due to the lower cell permeability of the ionized carboxylate group, in comparison of the molecules with the neutral moieties (52, 53). On the other side, the toxicity of the carboxyl-bearing compounds was approximately negligible at 10 μM concentration. Nevertheless, the previously-reported studies revealed that incorporating carboxylic moiety to the designed structures could enhance the solubility profile by forming carboxylate salts and improving bioavailability and physicochemical properties (54, 55). Hence, more studies are needed to improve the suitability of these compounds. Compounds 10, 11, and 16, containing an amidoxime function, with EC50 values of 25, 60.1, and 22 µM were identified as reasonable candidates for further investigations to find new anti-HIV hit compounds, especially considering the similar toxicity of AZT to these compounds. In contrast, compound 15 showed higher toxicity at 10 μM that could be due to the presence of two cyano groups in the molecule (56). Compound 13 with a group of chloromethyl has been designed and synthesized to detect the effects of adding an alkylating motif on anti-HIV activity and toxicity. Interestingly, despite the evidence of high cytotoxicity of alkylating agents, this compound displayed minor cytotoxic effects at a concentration of 10 μM and instead had good activity (EC50 = 17.9 μM). Taken together, our newly-developed compounds represent a promising scaffold for identifying a new group of triple-acting anti-HIV agents.
3.3. Molecular Modeling Studies
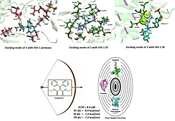
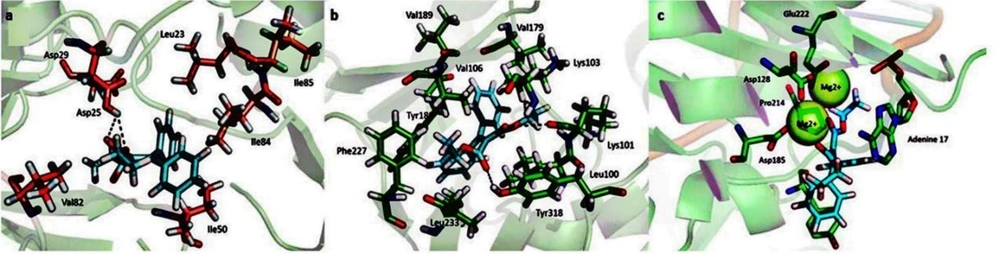
A docking study was performed to understand the binding mode of the newly designed ligands and rationalize the observed SAR. The prototype foamy virus (PFV) IN (PDB: 3OYA), a two-metal model for HIV IN in complex with the raltegravir (RAL), was used as a surrogate for an IN/viral DNA complex, as it explains the molecular interactions (57). Moreover, molecular docking into the RT and PR corresponding binding sites was carried out using the X-ray crystallographic structures of HIV RT (PDB ID: 4KO0) and PR (PDB ID: 1AJX), respectively. The docking poses and interactive sites of the compounds with amino acids are depicted in Figure 5. Docking results revealed that all compounds accommodated well in the binding sites with binding energies ranging from -5.7 to -10.1 kcal/mol (Figure 6). Most compounds demonstrated binding energies similar to the reference molecules, and in some compounds (8, 9, 11, 16), the binding energies to the RT enzyme were even better than that of the reference molecule. Compounds 4 and 16 indicated high binding affinities for HIV PR and RT binding sites with ∆G values of -8.4 and -10.1 kcal/mol, respectively. To further investigate the SAR for this series of derivatives, the best compound in cell-based HIV infection assay 3 was selected, and its binding to the enzymes’ binding sites was studied in more detail. The ethoxycarbonyl groups of 3 have been located in the 2.69, and 2.90 A° distance from Asp25 residue of the PR and involved in contact with enzyme through the hydrogen bonding interaction. The ethoxy group of the structure has been interacted via hydrophobic bonding with Val82 presenting in the active site. Moreover, the aryl groups were found to interact with Ile50 and Ile84 residues through hydrophobic bonding (Figure 5A). The docked pose of 3 into the RT allosteric site indicated that the diaryl moiety fitted in the hydrophobic pocket formed by Val106, Val179, Tyr188, Phe227, Leu234, and Pro236 residues.
Anti-HIV activity and docking results of the synthesized compounds (a the cellular toxicity was evaluated by XTT (sodium 3-[1(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid) proliferation assay (RocheTM, Germany); b Binding affinity of the novel compounds (ΔG) on the active site of the reverse transcriptase (RT), protease (PR), integrase (IN) enzymes were evaluated using crystallographic structures with PDB ID: 4KO0, PDB ID: 1AJX, PDB ID: 3OYA respectively; c Not determined; d Azidothymidine: reverse transcriptase inhibitor was used as a reference compound).
The double bond connected ethoxycarbonyl forms hydrogen bonds with the key residues Lys101 and Tyr318 of RT at average distances of 3.3 and 3.0 A°, respectively. Additionally, the vicinal aryl moieties interacted with Phe227 and Leu234 to form a hydrophobic bonding network (Figure 5B). Concerning the HIV IN active site, the ethoxycarbonyl moieties were found to coordinate with both the active site metal ions with distances of 2.04, 2.22, 2.26, and 2.33 Å (Figure 5C). Besides, the orientation of compound 3 in the active site shown in Figure 5C suggested that the phenyl ring involved in a π–stacking interaction with viral Tyr212. Potential hydrophobic interaction was also evident between the ethoxycarbonyl group and the base DA17. In addition, the other phenyl moiety occupied the hydrophobic pocket formed by Ala188, Ala189, and Prol214. The biological data were generally in agreement with the docking study, suggesting that their anti-HIV activity may be due to the triple RT/PR/IN inhibition. However, the observed orders in the two studies were not entirely the same, reflecting that factors other than the enzyme binding affinity are involved in determining the antiviral activity, especially in cell-based assays. Further investigation is needed to elucidate these factors and their possible role in the anti-HIV activity.
4. Conclusion
In summary, a series of novel 2-(diphenyl methylidene) malonic acid derivatives was designed as the triple HIV NNRT/IN/PR inhibitors. Docking analysis provided the probable binding modes and suggested that the newly-designed analogs bind with the respective enzyme active sites with proper affinities. The target molecules were synthesized in acceptable yields and structurally confirmed by analytical methods, including IR, LC-Mass, 1HNMR, 13CNMR, and elemental analysis. Notably, all target compounds exhibited no considerable cytotoxicity at the concentration of 10 μM in cell cultures. Moreover, most compounds displayed HIV inhibitory activity with EC50 values in a relatively low micromolar range. SAR analysis revealed compound 3 with two symmetrical parts in the “butterfly” conformation exhibited the best anti-HIV activity with an EC50 value of 8.4 μM. Besides, the determined toxicity profile of 3 supports this compound as a hit worthy of an anti-HIV agent for further investigation.
![Structures of the HIV-inhibitors from different antiretroviral drug classes [Indinavir: protease inhibitor (PI), Azidothymidine: nucleoside reverse transcriptase inhibitor (NRTI), Raltegravir: integrase inhibitor (INI), Nevirapine: non-nucleoside reverse transcriptase inhibitor (NNRTI)]. Structures of the HIV-inhibitors from different antiretroviral drug classes [Indinavir: protease inhibitor (PI), Azidothymidine: nucleoside reverse transcriptase inhibitor (NRTI), Raltegravir: integrase inhibitor (INI), Nevirapine: non-nucleoside reverse transcriptase inhibitor (NNRTI)].](https://services.brieflands.com/cdn/serve/3170b/116df626187d01d766820528ca14dea3cb64325c/ijpr-123827-i001-F1-preview.webp)
![Anti-HIV activity and docking results of the synthesized compounds (<sup>a</sup> the cellular toxicity was evaluated by XTT (sodium 3-[1(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid) proliferation assay (Roche<sup>TM</sup>, Germany); <sup>b</sup> Binding affinity of the novel compounds (ΔG) on the active site of the reverse transcriptase (RT), protease (PR), integrase (IN) enzymes were evaluated using crystallographic structures with PDB ID: 4KO0, PDB ID: 1AJX, PDB ID: 3OYA respectively; <sup>c</sup> Not determined; <sup>d</sup> Azidothymidine: reverse transcriptase inhibitor was used as a reference compound). Anti-HIV activity and docking results of the synthesized compounds (<sup>a</sup> the cellular toxicity was evaluated by XTT (sodium 3-[1(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid) proliferation assay (Roche<sup>TM</sup>, Germany); <sup>b</sup> Binding affinity of the novel compounds (ΔG) on the active site of the reverse transcriptase (RT), protease (PR), integrase (IN) enzymes were evaluated using crystallographic structures with PDB ID: 4KO0, PDB ID: 1AJX, PDB ID: 3OYA respectively; <sup>c</sup> Not determined; <sup>d</sup> Azidothymidine: reverse transcriptase inhibitor was used as a reference compound).](https://services.brieflands.com/cdn/serve/3170b/a7580627ce666d2da3d352a87b430c596d8f0286/ijpr-123827-i006-F6-preview.webp)